Annabel Sorby-Adams
From Low Field to High Value: Robust Cortical Mapping from Low-Field MRI
May 18, 2025Abstract:Three-dimensional reconstruction of cortical surfaces from MRI for morphometric analysis is fundamental for understanding brain structure. While high-field MRI (HF-MRI) is standard in research and clinical settings, its limited availability hinders widespread use. Low-field MRI (LF-MRI), particularly portable systems, offers a cost-effective and accessible alternative. However, existing cortical surface analysis tools are optimized for high-resolution HF-MRI and struggle with the lower signal-to-noise ratio and resolution of LF-MRI. In this work, we present a machine learning method for 3D reconstruction and analysis of portable LF-MRI across a range of contrasts and resolutions. Our method works "out of the box" without retraining. It uses a 3D U-Net trained on synthetic LF-MRI to predict signed distance functions of cortical surfaces, followed by geometric processing to ensure topological accuracy. We evaluate our method using paired HF/LF-MRI scans of the same subjects, showing that LF-MRI surface reconstruction accuracy depends on acquisition parameters, including contrast type (T1 vs T2), orientation (axial vs isotropic), and resolution. A 3mm isotropic T2-weighted scan acquired in under 4 minutes, yields strong agreement with HF-derived surfaces: surface area correlates at r=0.96, cortical parcellations reach Dice=0.98, and gray matter volume achieves r=0.93. Cortical thickness remains more challenging with correlations up to r=0.70, reflecting the difficulty of sub-mm precision with 3mm voxels. We further validate our method on challenging postmortem LF-MRI, demonstrating its robustness. Our method represents a step toward enabling cortical surface analysis on portable LF-MRI. Code is available at https://surfer.nmr.mgh.harvard.edu/fswiki/ReconAny
P-Count: Persistence-based Counting of White Matter Hyperintensities in Brain MRI
Mar 20, 2024Abstract:White matter hyperintensities (WMH) are a hallmark of cerebrovascular disease and multiple sclerosis. Automated WMH segmentation methods enable quantitative analysis via estimation of total lesion load, spatial distribution of lesions, and number of lesions (i.e., number of connected components after thresholding), all of which are correlated with patient outcomes. While the two former measures can generally be estimated robustly, the number of lesions is highly sensitive to noise and segmentation mistakes -- even when small connected components are eroded or disregarded. In this article, we present P-Count, an algebraic WMH counting tool based on persistent homology that accounts for the topological features of WM lesions in a robust manner. Using computational geometry, P-Count takes the persistence of connected components into consideration, effectively filtering out the noisy WMH positives, resulting in a more accurate count of true lesions. We validated P-Count on the ISBI2015 longitudinal lesion segmentation dataset, where it produces significantly more accurate results than direct thresholding.
PEPSI: Pathology-Enhanced Pulse-Sequence-Invariant Representations for Brain MRI
Mar 10, 2024



Abstract:Remarkable progress has been made by data-driven machine-learning methods in the analysis of MRI scans. However, most existing MRI analysis approaches are crafted for specific MR pulse sequences (MR contrasts) and usually require nearly isotropic acquisitions. This limits their applicability to diverse real-world clinical data, where scans commonly exhibit variations in appearances due to being obtained with varying sequence parameters, resolutions, and orientations -- especially in the presence of pathology. In this paper, we propose PEPSI, the first pathology-enhanced, and pulse-sequence-invariant feature representation learning model for brain MRI. PEPSI is trained entirely on synthetic images with a novel pathology encoding strategy, and enables co-training across datasets with diverse pathologies and missing modalities. Despite variations in pathology appearances across different MR pulse sequences or the quality of acquired images (e.g., resolution, orientation, artifacts, etc), PEPSI produces a high-resolution image of reference contrast (MP-RAGE) that captures anatomy, along with an image specifically highlighting the pathology. Our experiments demonstrate PEPSI's remarkable capability for image synthesis compared with the state-of-the-art, contrast-agnostic synthesis models, as it accurately reconstructs anatomical structures while differentiating between pathology and normal tissue. We further illustrate the efficiency and effectiveness of PEPSI features for downstream pathology segmentations on five public datasets covering white matter hyperintensities and stroke lesions. Code is available at https://github.com/peirong26/PEPSI.
Quantifying white matter hyperintensity and brain volumes in heterogeneous clinical and low-field portable MRI
Dec 08, 2023
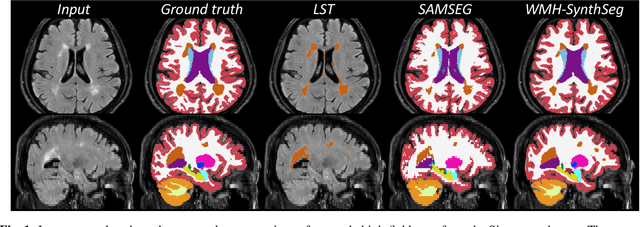
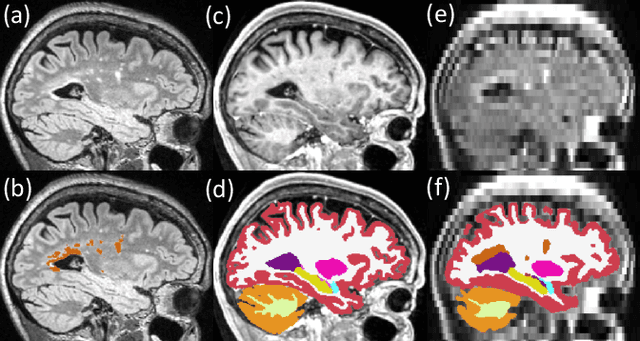

Abstract:Brain atrophy and white matter hyperintensity (WMH) are critical neuroimaging features for ascertaining brain injury in cerebrovascular disease and multiple sclerosis. Automated segmentation and quantification is desirable but existing methods require high-resolution MRI with good signal-to-noise ratio (SNR). This precludes application to clinical and low-field portable MRI (pMRI) scans, thus hampering large-scale tracking of atrophy and WMH progression, especially in underserved areas where pMRI has huge potential. Here we present a method that segments white matter hyperintensity and 36 brain regions from scans of any resolution and contrast (including pMRI) without retraining. We show results on six public datasets and on a private dataset with paired high- and low-field scans (3T and 64mT), where we attain strong correlation between the WMH ($\rho$=.85) and hippocampal volumes (r=.89) estimated at both fields. Our method is publicly available as part of FreeSurfer, at: http://surfer.nmr.mgh.harvard.edu/fswiki/WMH-SynthSeg.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge