Anna D'Angelo
To deform or not: treatment-aware longitudinal registration for breast DCE-MRI during neoadjuvant chemotherapy via unsupervised keypoints detection
Jan 17, 2024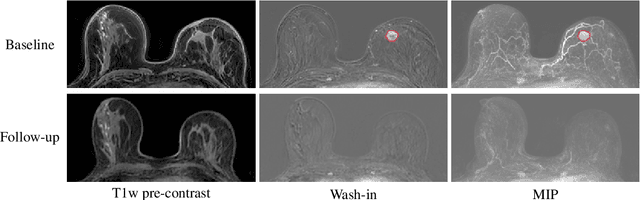
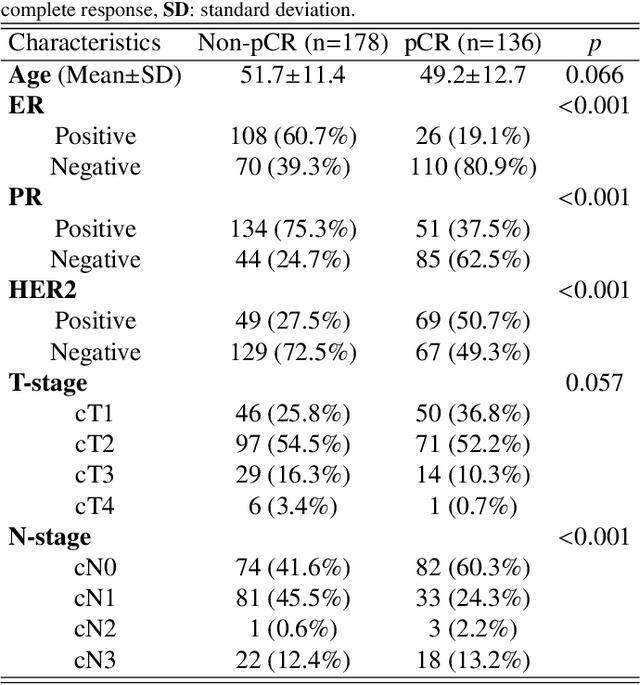
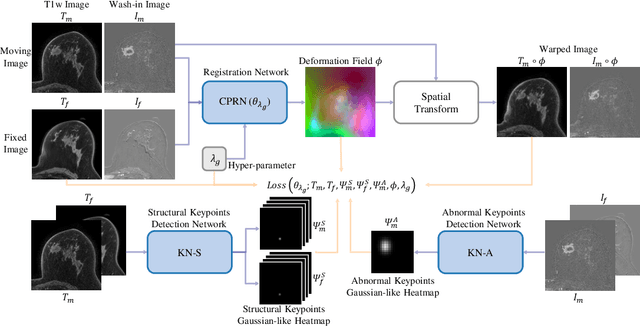
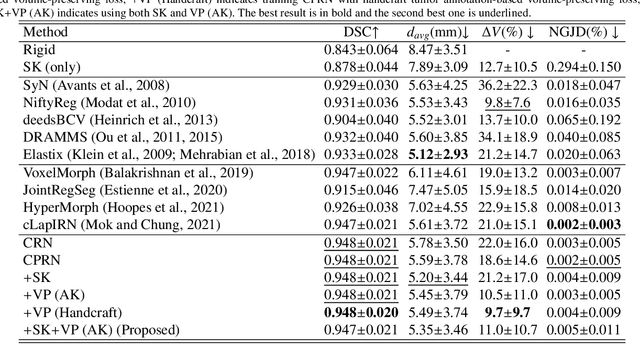
Abstract:Clinicians compare breast DCE-MRI after neoadjuvant chemotherapy (NAC) with pre-treatment scans to evaluate the response to NAC. Clinical evidence supports that accurate longitudinal deformable registration without deforming treated tumor regions is key to quantifying tumor changes. We propose a conditional pyramid registration network based on unsupervised keypoint detection and selective volume-preserving to quantify changes over time. In this approach, we extract the structural and the abnormal keypoints from DCE-MRI, apply the structural keypoints for the registration algorithm to restrict large deformation, and employ volume-preserving loss based on abnormal keypoints to keep the volume of the tumor unchanged after registration. We use a clinical dataset with 1630 MRI scans from 314 patients treated with NAC. The results demonstrate that our method registers with better performance and better volume preservation of the tumors. Furthermore, a local-global-combining biomarker based on the proposed method achieves high accuracy in pathological complete response (pCR) prediction, indicating that predictive information exists outside tumor regions. The biomarkers could potentially be used to avoid unnecessary surgeries for certain patients. It may be valuable for clinicians and/or computer systems to conduct follow-up tumor segmentation and response prediction on images registered by our method. Our code is available on \url{https://github.com/fiy2W/Treatment-aware-Longitudinal-Registration}.
Synthesis of Contrast-Enhanced Breast MRI Using Multi-b-Value DWI-based Hierarchical Fusion Network with Attention Mechanism
Jul 03, 2023



Abstract:Magnetic resonance imaging (MRI) is the most sensitive technique for breast cancer detection among current clinical imaging modalities. Contrast-enhanced MRI (CE-MRI) provides superior differentiation between tumors and invaded healthy tissue, and has become an indispensable technique in the detection and evaluation of cancer. However, the use of gadolinium-based contrast agents (GBCA) to obtain CE-MRI may be associated with nephrogenic systemic fibrosis and may lead to bioaccumulation in the brain, posing a potential risk to human health. Moreover, and likely more important, the use of gadolinium-based contrast agents requires the cannulation of a vein, and the injection of the contrast media which is cumbersome and places a burden on the patient. To reduce the use of contrast agents, diffusion-weighted imaging (DWI) is emerging as a key imaging technique, although currently usually complementing breast CE-MRI. In this study, we develop a multi-sequence fusion network to synthesize CE-MRI based on T1-weighted MRI and DWIs. DWIs with different b-values are fused to efficiently utilize the difference features of DWIs. Rather than proposing a pure data-driven approach, we invent a multi-sequence attention module to obtain refined feature maps, and leverage hierarchical representation information fused at different scales while utilizing the contributions from different sequences from a model-driven approach by introducing the weighted difference module. The results show that the multi-b-value DWI-based fusion model can potentially be used to synthesize CE-MRI, thus theoretically reducing or avoiding the use of GBCA, thereby minimizing the burden to patients. Our code is available at \url{https://github.com/Netherlands-Cancer-Institute/CE-MRI}.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge