Anca L. Ralescu
Joint Self-Supervised and Supervised Contrastive Learning for Multimodal MRI Data: Towards Predicting Abnormal Neurodevelopment
Dec 22, 2023



Abstract:The integration of different imaging modalities, such as structural, diffusion tensor, and functional magnetic resonance imaging, with deep learning models has yielded promising outcomes in discerning phenotypic characteristics and enhancing disease diagnosis. The development of such a technique hinges on the efficient fusion of heterogeneous multimodal features, which initially reside within distinct representation spaces. Naively fusing the multimodal features does not adequately capture the complementary information and could even produce redundancy. In this work, we present a novel joint self-supervised and supervised contrastive learning method to learn the robust latent feature representation from multimodal MRI data, allowing the projection of heterogeneous features into a shared common space, and thereby amalgamating both complementary and analogous information across various modalities and among similar subjects. We performed a comparative analysis between our proposed method and alternative deep multimodal learning approaches. Through extensive experiments on two independent datasets, the results demonstrated that our method is significantly superior to several other deep multimodal learning methods in predicting abnormal neurodevelopment. Our method has the capability to facilitate computer-aided diagnosis within clinical practice, harnessing the power of multimodal data.
Learning Empirical Bregman Divergence for Uncertain Distance Representation
Apr 18, 2023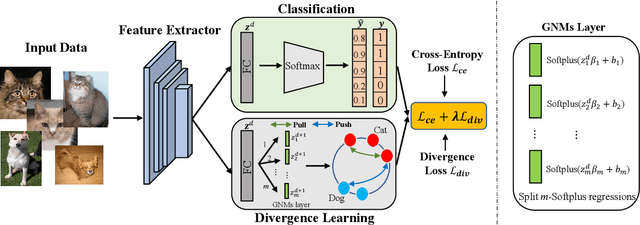
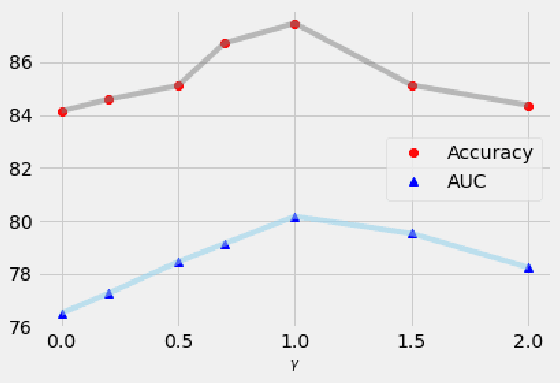
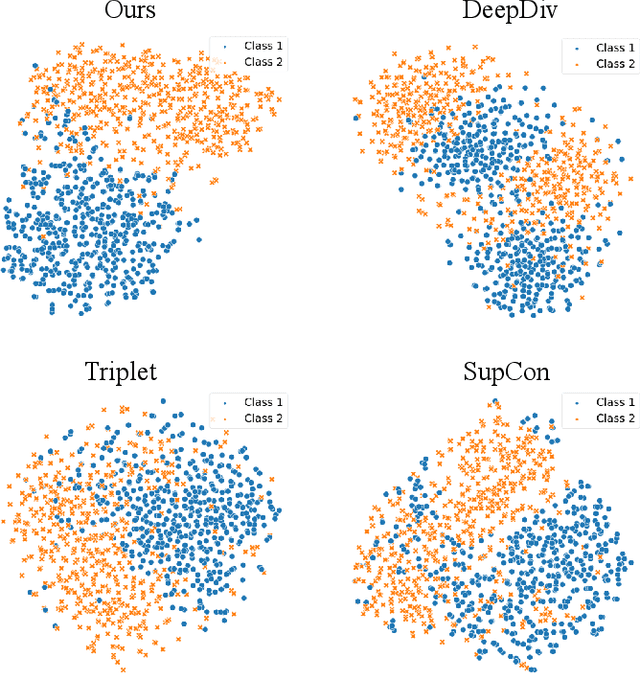

Abstract:Deep metric learning techniques have been used for visual representation in various supervised and unsupervised learning tasks through learning embeddings of samples with deep networks. However, classic approaches, which employ a fixed distance metric as a similarity function between two embeddings, may lead to suboptimal performance for capturing the complex data distribution. The Bregman divergence generalizes measures of various distance metrics and arises throughout many fields of deep metric learning. In this paper, we first show how deep metric learning loss can arise from the Bregman divergence. We then introduce a novel method for learning empirical Bregman divergence directly from data based on parameterizing the convex function underlying the Bregman divergence with a deep learning setting. We further experimentally show that our approach performs effectively on five popular public datasets compared to other SOTA deep metric learning methods, particularly for pattern recognition problems.
A Novel Collaborative Self-Supervised Learning Method for Radiomic Data
Feb 20, 2023



Abstract:The computer-aided disease diagnosis from radiomic data is important in many medical applications. However, developing such a technique relies on annotating radiological images, which is a time-consuming, labor-intensive, and expensive process. In this work, we present the first novel collaborative self-supervised learning method to solve the challenge of insufficient labeled radiomic data, whose characteristics are different from text and image data. To achieve this, we present two collaborative pretext tasks that explore the latent pathological or biological relationships between regions of interest and the similarity and dissimilarity information between subjects. Our method collaboratively learns the robust latent feature representations from radiomic data in a self-supervised manner to reduce human annotation efforts, which benefits the disease diagnosis. We compared our proposed method with other state-of-the-art self-supervised learning methods on a simulation study and two independent datasets. Extensive experimental results demonstrated that our method outperforms other self-supervised learning methods on both classification and regression tasks. With further refinement, our method shows the potential advantage in automatic disease diagnosis with large-scale unlabeled data available.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge