Alex Ling Yu Hung
Cross-Slice Attention and Evidential Critical Loss for Uncertainty-Aware Prostate Cancer Detection
Jul 01, 2024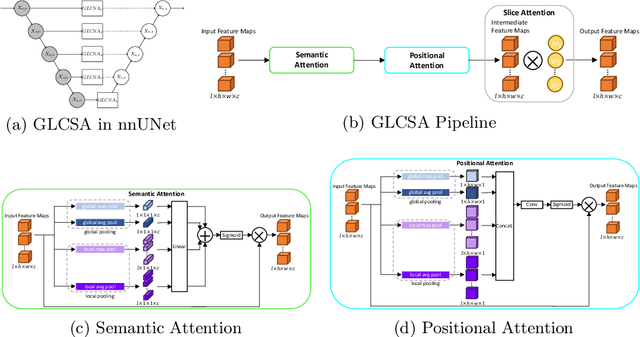

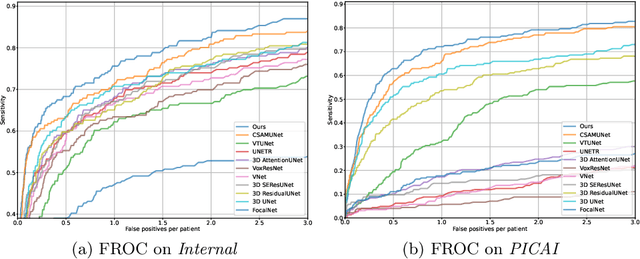
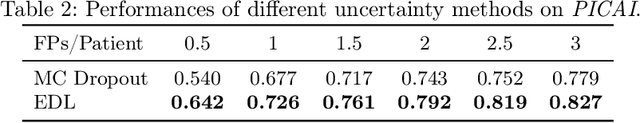
Abstract:Current deep learning-based models typically analyze medical images in either 2D or 3D albeit disregarding volumetric information or suffering sub-optimal performance due to the anisotropic resolution of MR data. Furthermore, providing an accurate uncertainty estimation is beneficial to clinicians, as it indicates how confident a model is about its prediction. We propose a novel 2.5D cross-slice attention model that utilizes both global and local information, along with an evidential critical loss, to perform evidential deep learning for the detection in MR images of prostate cancer, one of the most common cancers and a leading cause of cancer-related death in men. We perform extensive experiments with our model on two different datasets and achieve state-of-the-art performance in prostate cancer detection along with improved epistemic uncertainty estimation. The implementation of the model is available at https://github.com/aL3x-O-o-Hung/GLCSA_ECLoss.
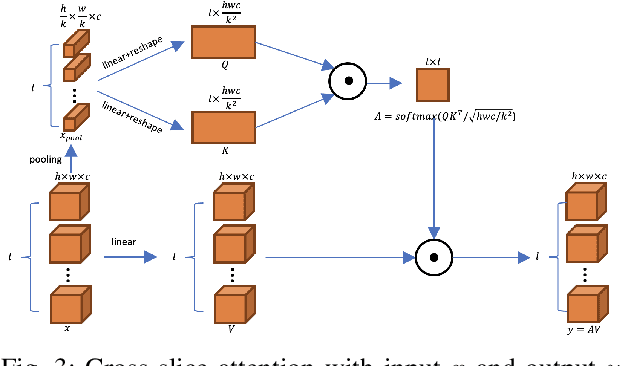
CSAM: A 2.5D Cross-Slice Attention Module for Anisotropic Volumetric Medical Image Segmentation
Nov 08, 2023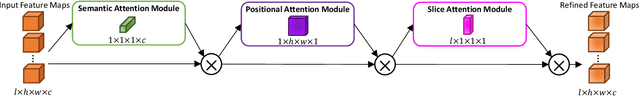
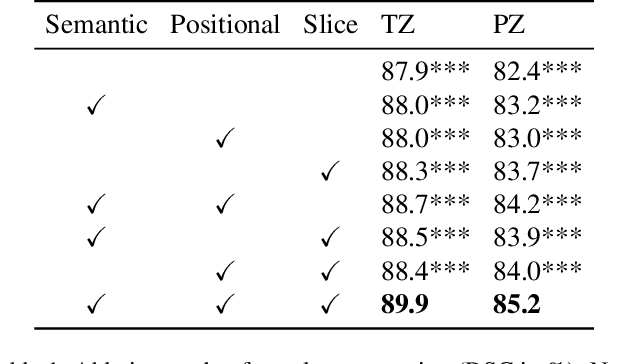
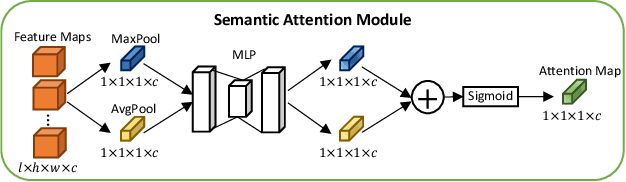
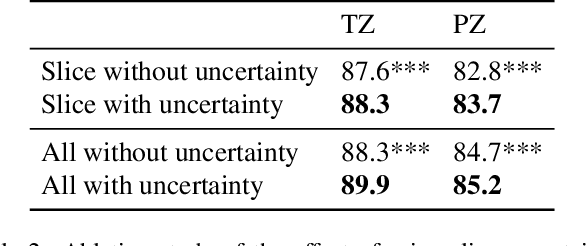
Abstract:A large portion of volumetric medical data, especially magnetic resonance imaging (MRI) data, is anisotropic, as the through-plane resolution is typically much lower than the in-plane resolution. Both 3D and purely 2D deep learning-based segmentation methods are deficient in dealing with such volumetric data since the performance of 3D methods suffers when confronting anisotropic data, and 2D methods disregard crucial volumetric information. Insufficient work has been done on 2.5D methods, in which 2D convolution is mainly used in concert with volumetric information. These models focus on learning the relationship across slices, but typically have many parameters to train. We offer a Cross-Slice Attention Module (CSAM) with minimal trainable parameters, which captures information across all the slices in the volume by applying semantic, positional, and slice attention on deep feature maps at different scales. Our extensive experiments using different network architectures and tasks demonstrate the usefulness and generalizability of CSAM. Associated code is available at https://github.com/aL3x-O-o-Hung/CSAM.
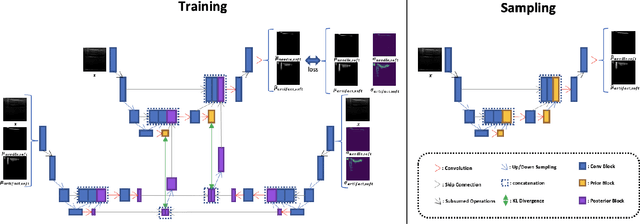
PartDiff: Image Super-resolution with Partial Diffusion Models
Jul 21, 2023Abstract:Denoising diffusion probabilistic models (DDPMs) have achieved impressive performance on various image generation tasks, including image super-resolution. By learning to reverse the process of gradually diffusing the data distribution into Gaussian noise, DDPMs generate new data by iteratively denoising from random noise. Despite their impressive performance, diffusion-based generative models suffer from high computational costs due to the large number of denoising steps.In this paper, we first observed that the intermediate latent states gradually converge and become indistinguishable when diffusing a pair of low- and high-resolution images. This observation inspired us to propose the Partial Diffusion Model (PartDiff), which diffuses the image to an intermediate latent state instead of pure random noise, where the intermediate latent state is approximated by the latent of diffusing the low-resolution image. During generation, Partial Diffusion Models start denoising from the intermediate distribution and perform only a part of the denoising steps. Additionally, to mitigate the error caused by the approximation, we introduce "latent alignment", which aligns the latent between low- and high-resolution images during training. Experiments on both magnetic resonance imaging (MRI) and natural images show that, compared to plain diffusion-based super-resolution methods, Partial Diffusion Models significantly reduce the number of denoising steps without sacrificing the quality of generation.
CAT-Net: A Cross-Slice Attention Transformer Model for Prostate Zonal Segmentation in MRI
Mar 29, 2022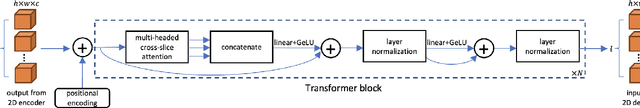
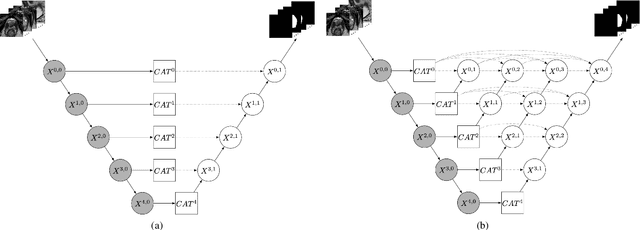
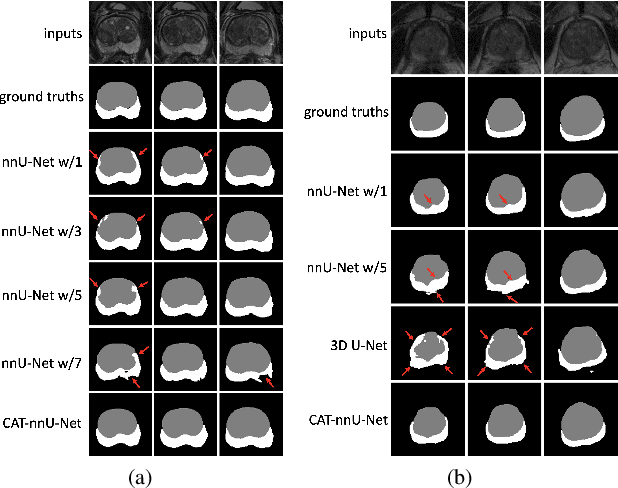
Abstract:Prostate cancer is the second leading cause of cancer death among men in the United States. The diagnosis of prostate MRI often relies on the accurate prostate zonal segmentation. However, state-of-the-art automatic segmentation methods often fail to produce well-contained volumetric segmentation of the prostate zones since certain slices of prostate MRI, such as base and apex slices, are harder to segment than other slices. This difficulty can be overcome by accounting for the cross-slice relationship of adjacent slices, but current methods do not fully learn and exploit such relationships. In this paper, we propose a novel cross-slice attention mechanism, which we use in a Transformer module to systematically learn the cross-slice relationship at different scales. The module can be utilized in any existing learning-based segmentation framework with skip connections. Experiments show that our cross-slice attention is able to capture the cross-slice information in prostate zonal segmentation and improve the performance of current state-of-the-art methods. Our method significantly improves segmentation accuracy in the peripheral zone, such that the segmentation results are consistent across all the prostate slices (apex, mid-gland, and base).
The Role of Pleura and Adipose in Lung Ultrasound AI
Jan 19, 2022Abstract:In this paper, we study the significance of the pleura and adipose tissue in lung ultrasound AI analysis. We highlight their more prominent appearance when using high-frequency linear (HFL) instead of curvilinear ultrasound probes, showing HFL reveals better pleura detail. We compare the diagnostic utility of the pleura and adipose tissue using an HFL ultrasound probe. Masking the adipose tissue during training and inference (while retaining the pleural line and Merlin's space artifacts such as A-lines and B-lines) improved the AI model's diagnostic accuracy.
* Published in MICCAI 2021 workshop on Lessons Learned from the development and application of medical imaging-based AI technologies for combating COVID-19 (LL-COVID19). The first two authors contributed equally to this work
Good and Bad Boundaries in Ultrasound Compounding: Preserving Anatomic Boundaries While Suppressing Artifacts
Nov 24, 2020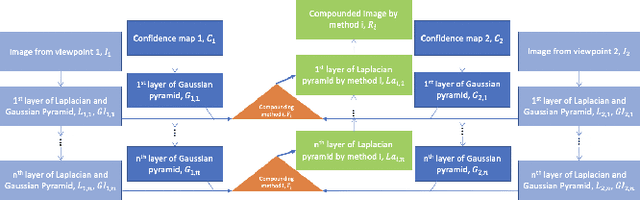
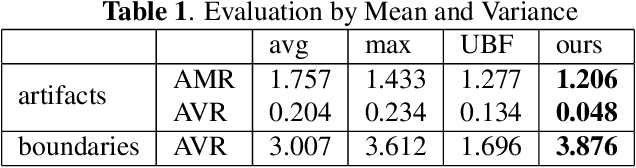
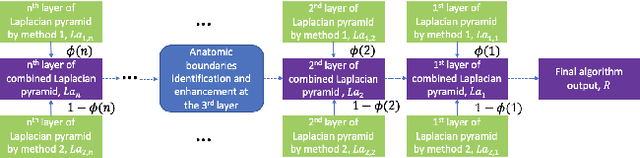

Abstract:Ultrasound 3D compounding is important for volumetric reconstruction, but as of yet there is no consensus on best practices for compounding. Ultrasound images depend on probe direction and the path sound waves pass through, so when multiple intersecting B-scans of the same spot from different perspectives yield different pixel values, there is not a single, ideal representation for compounding (i.e. combining) the overlapping pixel values. Current popular methods inevitably suppress or altogether leave out bright or dark regions that are useful, and potentially introduce new artifacts. In this work, we establish a new algorithm to compound the overlapping pixels from different view points in ultrasound. We uniquely leverage Laplacian and Gaussian Pyramids to preserve the maximum boundary contrast without overemphasizing noise and speckle. We evaluate our algorithm by comparing ours with previous algorithms, and we show that our approach not only preserves both light and dark details, but also somewhat suppresses artifacts, rather than amplifying them.
Weakly- and Semi-Supervised Probabilistic Segmentation and Quantification of Ultrasound Needle-Reverberation Artifacts to Allow Better AI Understanding of Tissue Beneath Needles
Nov 24, 2020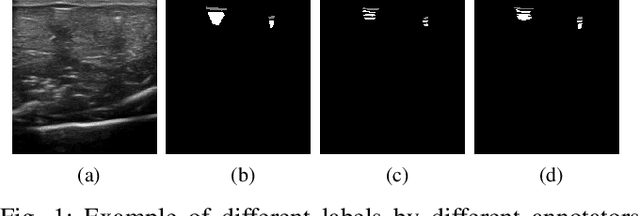
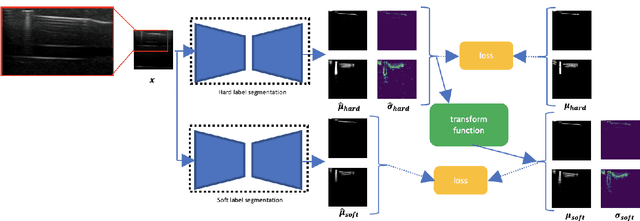
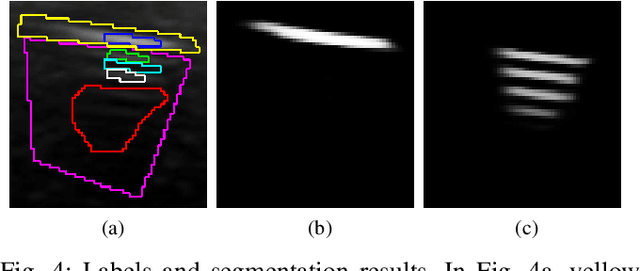
Abstract:Ultrasound image quality has been continually improving. However, when needles or other metallic objects are operating inside the tissue, the resulting reverberation artifacts can severely corrupt the surrounding image quality. Such effects are challenging for existing computer vision algorithms for medical image analysis. Needle reverberation artifacts can be hard to identify at times and affect various pixel values to different degrees. The boundaries of such artifacts are ambiguous, leading to disagreement among human experts labeling the artifacts. We purpose a weakly- and semi-supervised, probabilistic needle-and-needle-artifact segmentation algorithm to separate the desired tissue-based pixel values from the superimposed artifacts. Our method models the intensity decay of artifact intensities and is designed to minimize the human labeling error. We demonstrate the applicability of the approach, comparing it against other segmentation algorithms. Our method is capable of differentiating the reverberations from artifact-free patches between reverberations, as well as modeling the intensity fall-off in the artifacts. Our method matches state-of-the-art artifact segmentation performance, and sets a new standard in estimating the per-pixel contributions of artifact vs underlying anatomy, especially in the immediately adjacent regions between reverberation lines.
Ultrasound Confidence Maps of Intensity and Structure Based on Directed Acyclic Graphs and Artifact Models
Nov 24, 2020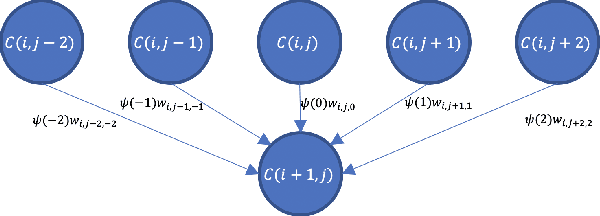
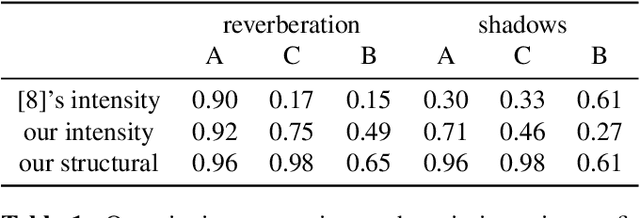
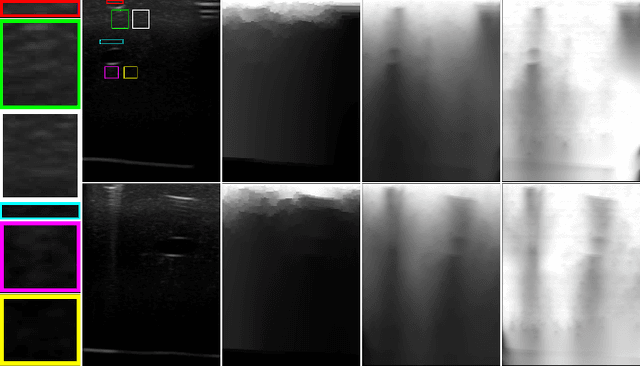
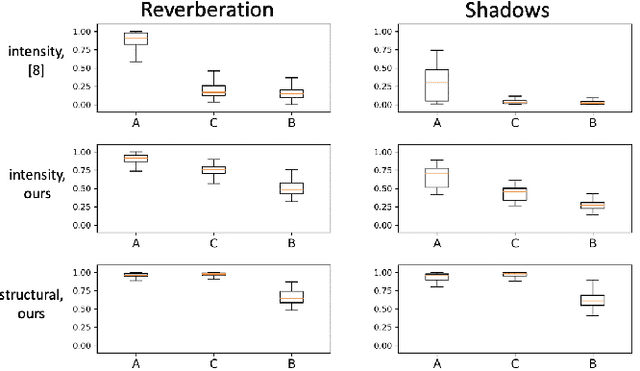
Abstract:Ultrasound imaging has been improving, but continues to suffer from inherent artifacts that are challenging to model, such as attenuation, shadowing, diffraction, speckle, etc. These artifacts can potentially confuse image analysis algorithms unless an attempt is made to assess the certainty of individual pixel values. Our novel confidence algorithms analyze pixel values using a directed acyclic graph based on acoustic physical properties of ultrasound imaging. We demonstrate unique capabilities of our approach and compare it against previous confidence-measurement algorithms for shadow-detection and image-compounding tasks.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge