Aldo Marzullo
Exploring Zero-Shot Anomaly Detection with CLIP in Medical Imaging: Are We There Yet?
Nov 14, 2024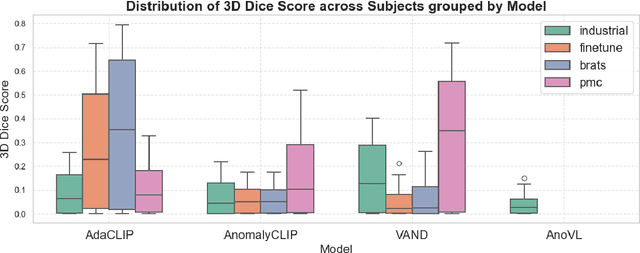
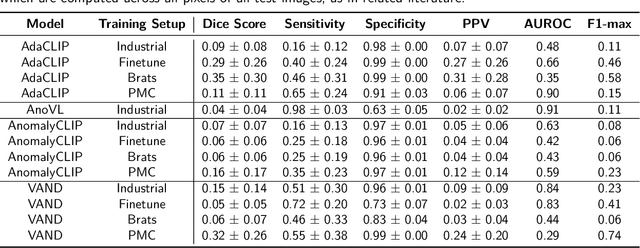
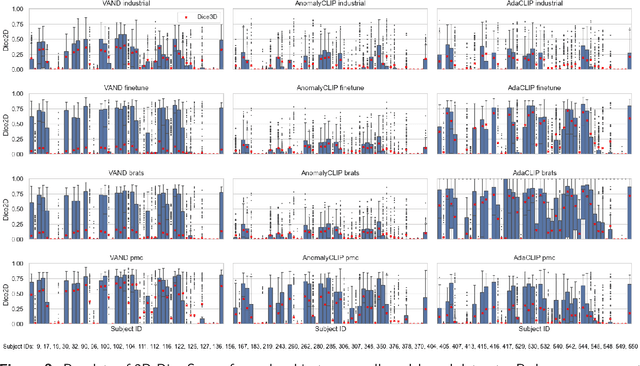
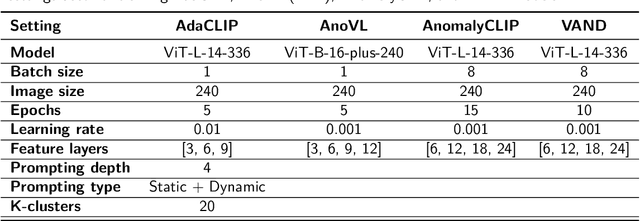
Abstract:Zero-shot anomaly detection (ZSAD) offers potential for identifying anomalies in medical imaging without task-specific training. In this paper, we evaluate CLIP-based models, originally developed for industrial tasks, on brain tumor detection using the BraTS-MET dataset. Our analysis examines their ability to detect medical-specific anomalies with no or minimal supervision, addressing the challenges posed by limited data annotation. While these models show promise in transferring general knowledge to medical tasks, their performance falls short of the precision required for clinical use. Our findings highlight the need for further adaptation before CLIP-based models can be reliably applied to medical anomaly detection.
A transfer-learning approach for lesion detection in endoscopic images from the urinary tract
Apr 08, 2021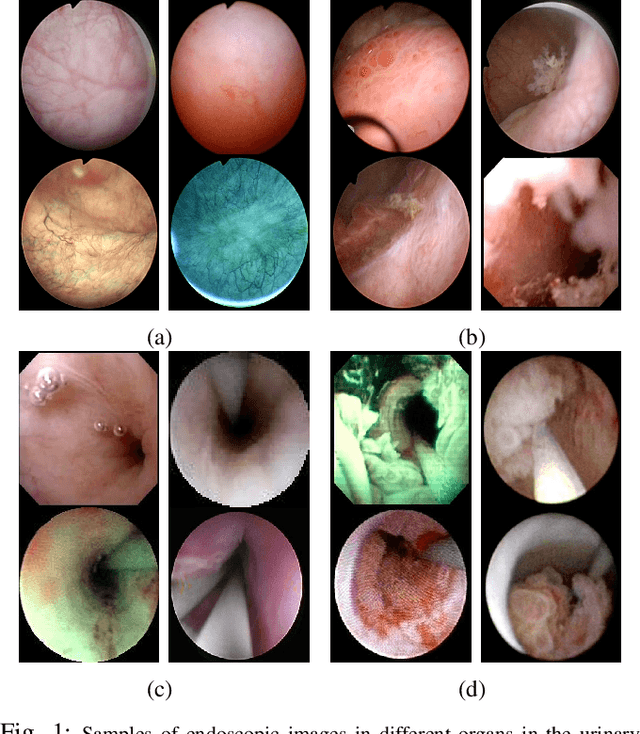
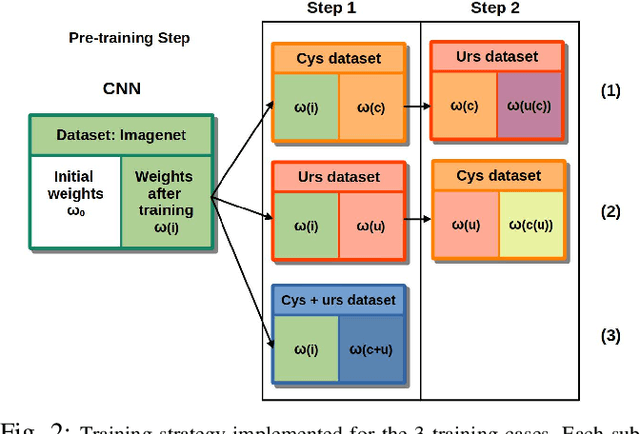
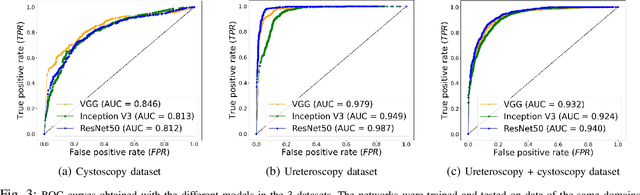
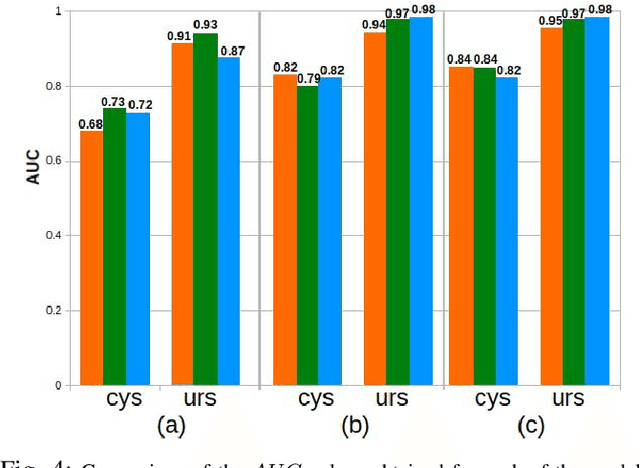
Abstract:Ureteroscopy and cystoscopy are the gold standard methods to identify and treat tumors along the urinary tract. It has been reported that during a normal procedure a rate of 10-20 % of the lesions could be missed. In this work we study the implementation of 3 different Convolutional Neural Networks (CNNs), using a 2-steps training strategy, to classify images from the urinary tract with and without lesions. A total of 6,101 images from ureteroscopy and cystoscopy procedures were collected. The CNNs were trained and tested using transfer learning in a two-steps fashion on 3 datasets. The datasets used were: 1) only ureteroscopy images, 2) only cystoscopy images and 3) the combination of both of them. For cystoscopy data, VGG performed better obtaining an Area Under the ROC Curve (AUC) value of 0.846. In the cases of ureteroscopy and the combination of both datasets, ResNet50 achieved the best results with AUC values of 0.987 and 0.940. The use of a training dataset that comprehends both domains results in general better performances, but performing a second stage of transfer learning achieves comparable ones. There is no single model which performs better in all scenarios, but ResNet50 is the network that achieves the best performances in most of them. The obtained results open the opportunity for further investigation with a view for improving lesion detection in endoscopic images of the urinary system.
Using spatial-temporal ensembles of convolutional neural networks for lumen segmentation in ureteroscopy
Apr 05, 2021



Abstract:Purpose: Ureteroscopy is an efficient endoscopic minimally invasive technique for the diagnosis and treatment of upper tract urothelial carcinoma (UTUC). During ureteroscopy, the automatic segmentation of the hollow lumen is of primary importance, since it indicates the path that the endoscope should follow. In order to obtain an accurate segmentation of the hollow lumen, this paper presents an automatic method based on Convolutional Neural Networks (CNNs). Methods: The proposed method is based on an ensemble of 4 parallel CNNs to simultaneously process single and multi-frame information. Of these, two architectures are taken as core-models, namely U-Net based in residual blocks($m_1$) and Mask-RCNN($m_2$), which are fed with single still-frames $I(t)$. The other two models ($M_1$, $M_2$) are modifications of the former ones consisting on the addition of a stage which makes use of 3D Convolutions to process temporal information. $M_1$, $M_2$ are fed with triplets of frames ($I(t-1)$, $I(t)$, $I(t+1)$) to produce the segmentation for $I(t)$. Results: The proposed method was evaluated using a custom dataset of 11 videos (2,673 frames) which were collected and manually annotated from 6 patients. We obtain a Dice similarity coefficient of 0.80, outperforming previous state-of-the-art methods. Conclusion: The obtained results show that spatial-temporal information can be effectively exploited by the ensemble model to improve hollow lumen segmentation in ureteroscopic images. The method is effective also in presence of poor visibility, occasional bleeding, or specular reflections.
A Lumen Segmentation Method in Ureteroscopy Images based on a Deep Residual U-Net architecture
Jan 13, 2021



Abstract:Ureteroscopy is becoming the first surgical treatment option for the majority of urinary affections. This procedure is performed using an endoscope which provides the surgeon with the visual information necessary to navigate inside the urinary tract. Having in mind the development of surgical assistance systems, that could enhance the performance of surgeon, the task of lumen segmentation is a fundamental part since this is the visual reference which marks the path that the endoscope should follow. This is something that has not been analyzed in ureteroscopy data before. However, this task presents several challenges given the image quality and the conditions itself of ureteroscopy procedures. In this paper, we study the implementation of a Deep Neural Network which exploits the advantage of residual units in an architecture based on U-Net. For the training of these networks, we analyze the use of two different color spaces: gray-scale and RGB data images. We found that training on gray-scale images gives the best results obtaining mean values of Dice Score, Precision, and Recall of 0.73, 0.58, and 0.92 respectively. The results obtained shows that the use of residual U-Net could be a suitable model for further development for a computer-aided system for navigation and guidance through the urinary system.
A Logic-Based Framework Leveraging Neural Networks for Studying the Evolution of Neurological Disorders
Oct 21, 2019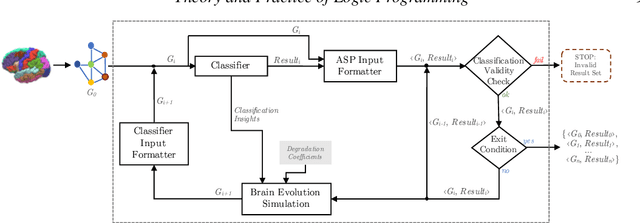
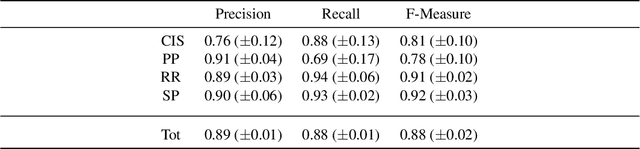
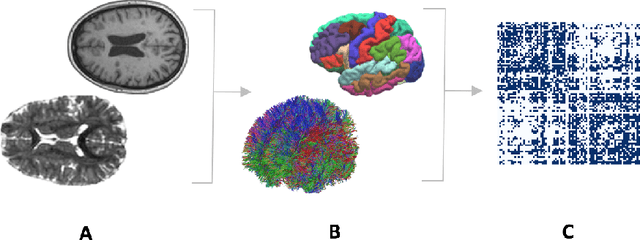
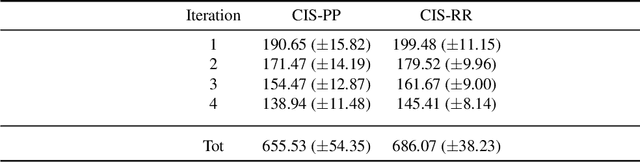
Abstract:Deductive formalisms have been strongly developed in recent years; among them, Answer Set Programming (ASP) gained some momentum, and has been lately fruitfully employed in many real-world scenarios. Nonetheless, in spite of a large number of success stories in relevant application areas, and even in industrial contexts, deductive reasoning cannot be considered the ultimate, comprehensive solution to AI; indeed, in several contexts, other approaches result to be more useful. Typical Bioinformatics tasks, for instance classification, are currently carried out mostly by Machine Learning (ML) based solutions. In this paper, we focus on the relatively new problem of analyzing the evolution of neurological disorders. In this context, ML approaches already demonstrated to be a viable solution for classification tasks; here, we show how ASP can play a relevant role in the brain evolution simulation task. In particular, we propose a general and extensible framework to support physicians and researchers at understanding the complex mechanisms underlying neurological disorders. The framework relies on a combined use of ML and ASP, and is general enough to be applied in several other application scenarios, which are outlined in the paper.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge