Adnan Saeed
DACB-Net: Dual Attention Guided Compact Bilinear Convolution Neural Network for Skin Disease Classification
Jul 03, 2024
Abstract:This paper introduces the three-branch Dual Attention-Guided Compact Bilinear CNN (DACB-Net) by focusing on learning from disease-specific regions to enhance accuracy and alignment. A global branch compensates for lost discriminative features, generating Attention Heat Maps (AHM) for relevant cropped regions. Finally, the last pooling layers of global and local branches are concatenated for fine-tuning, which offers a comprehensive solution to the challenges posed by skin disease diagnosis. Although current CNNs employ Stochastic Gradient Descent (SGD) for discriminative feature learning, using distinct pairs of local image patches to compute gradients and incorporating a modulation factor in the loss for focusing on complex data during training. However, this approach can lead to dataset imbalance, weight adjustments, and vulnerability to overfitting. The proposed solution combines two supervision branches and a novel loss function to address these issues, enhancing performance and interpretability. The framework integrates data augmentation, transfer learning, and fine-tuning to tackle data imbalance to improve classification performance, and reduce computational costs. Simulations on the HAM10000 and ISIC2019 datasets demonstrate the effectiveness of this approach, showcasing a 2.59% increase in accuracy compared to the state-of-the-art.
Bilinear-Convolutional Neural Network Using a Matrix Similarity-based Joint Loss Function for Skin Disease Classification
Jun 02, 2024


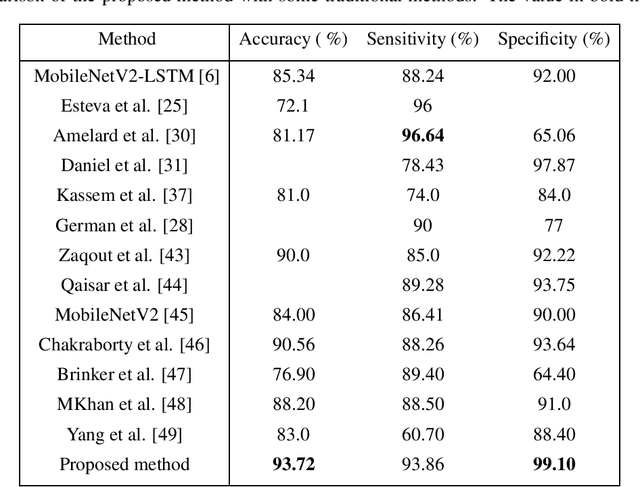
Abstract:In this study, we proposed a model for skin disease classification using a Bilinear Convolutional Neural Network (BCNN) with a Constrained Triplet Network (CTN). BCNN can capture rich spatial interactions between features in image data. This computes the outer product of feature vectors from two different CNNs by a bilinear pooling. The resulting features encode second-order statistics, enabling the network to capture more complex relationships between different channels and spatial locations. The CTN employs the Triplet Loss Function (TLF) by using a new loss layer that is added at the end of the architecture called the Constrained Triplet Loss (CTL) layer. This is done to obtain two significant learning objectives: inter-class categorization and intra-class concentration with their deep features as often as possible, which can be effective for skin disease classification. The proposed model is trained to extract the intra-class features from a deep network and accordingly increases the distance between these features, improving the model's performance. The model achieved a mean accuracy of 93.72%.
Context Aware 3D UNet for Brain Tumor Segmentation
Oct 25, 2020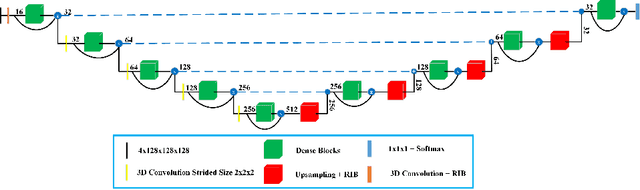
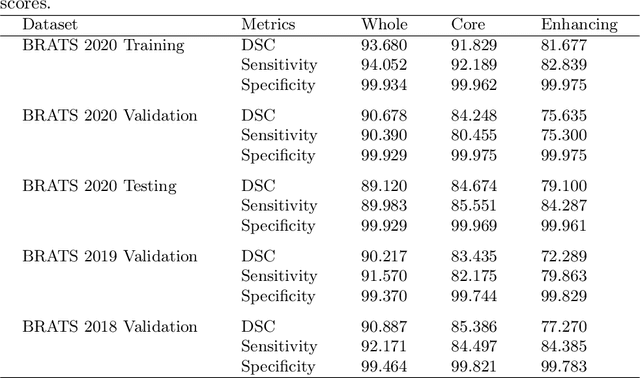
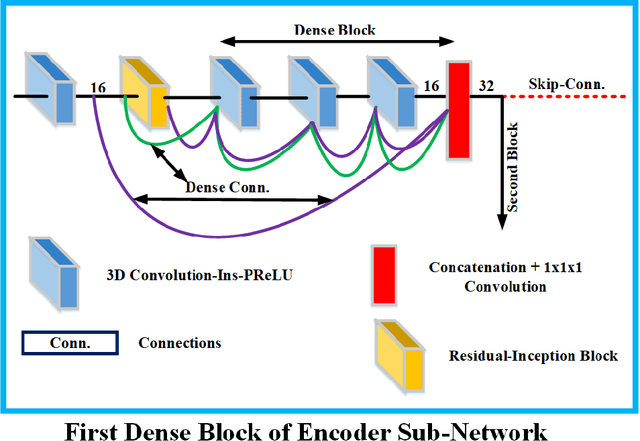
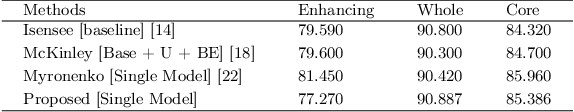
Abstract:Deep convolutional neural network (CNN) achieves remarkable performance for medical image analysis. UNet is the primary source in the performance of 3D CNN architectures for medical imaging tasks, including brain tumor segmentation. The skip connection in the UNet architecture concatenates features from both encoder and decoder paths to extract multi-contexual information from image data. The multi-scaled features play an essential role in brain tumor segmentation. However, the limited use of features can degrade the performance of the UNet approach for segmentation. In this paper, we propose a modified UNet architecture for brain tumor segmentation. In the proposed architecture, we used densely connected blocks in both encoder and decoder paths to extract multi-contexual information from the concept of feature reusability. The proposed residual inception blocks (RIB) are used to extract local and global information by merging features of different kernel sizes. We validate the proposed architecture on the multimodal brain tumor segmentation challenges (BRATS) 2020 testing dataset. The dice (DSC) scores of the whole tumor (WT), tumor core (TC), and enhancement tumor (ET) are 89.12%, 84.74%, and 79.12%, respectively. Our proposed work is in the top ten methods based on the dice scores of the testing dataset.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge