Abhilash Rakkunedeth Hareendranathan
A Simple Framework Uniting Visual In-context Learning with Masked Image Modeling to Improve Ultrasound Segmentation
Mar 08, 2024



Abstract:Conventional deep learning models deal with images one-by-one, requiring costly and time-consuming expert labeling in the field of medical imaging, and domain-specific restriction limits model generalizability. Visual in-context learning (ICL) is a new and exciting area of research in computer vision. Unlike conventional deep learning, ICL emphasizes the model's ability to adapt to new tasks based on given examples quickly. Inspired by MAE-VQGAN, we proposed a new simple visual ICL method called SimICL, combining visual ICL pairing images with masked image modeling (MIM) designed for self-supervised learning. We validated our method on bony structures segmentation in a wrist ultrasound (US) dataset with limited annotations, where the clinical objective was to segment bony structures to help with further fracture detection. We used a test set containing 3822 images from 18 patients for bony region segmentation. SimICL achieved an remarkably high Dice coeffient (DC) of 0.96 and Jaccard Index (IoU) of 0.92, surpassing state-of-the-art segmentation and visual ICL models (a maximum DC 0.86 and IoU 0.76), with SimICL DC and IoU increasing up to 0.10 and 0.16. This remarkably high agreement with limited manual annotations indicates SimICL could be used for training AI models even on small US datasets. This could dramatically decrease the human expert time required for image labeling compared to conventional approaches, and enhance the real-world use of AI assistance in US image analysis.
Self-supervised TransUNet for Ultrasound regional segmentation of the distal radius in children
Sep 18, 2023



Abstract:Supervised deep learning offers great promise to automate analysis of medical images from segmentation to diagnosis. However, their performance highly relies on the quality and quantity of the data annotation. Meanwhile, curating large annotated datasets for medical images requires a high level of expertise, which is time-consuming and expensive. Recently, to quench the thirst for large data sets with high-quality annotation, self-supervised learning (SSL) methods using unlabeled domain-specific data, have attracted attention. Therefore, designing an SSL method that relies on minimal quantities of labeled data has far-reaching significance in medical images. This paper investigates the feasibility of deploying the Masked Autoencoder for SSL (SSL-MAE) of TransUNet, for segmenting bony regions from children's wrist ultrasound scans. We found that changing the embedding and loss function in SSL-MAE can produce better downstream results compared to the original SSL-MAE. In addition, we determined that only pretraining TransUNet embedding and encoder with SSL-MAE does not work as well as TransUNet without SSL-MAE pretraining on downstream segmentation tasks.
Sample Efficient Learning of Image-Based Diagnostic Classifiers Using Probabilistic Labels
Feb 11, 2021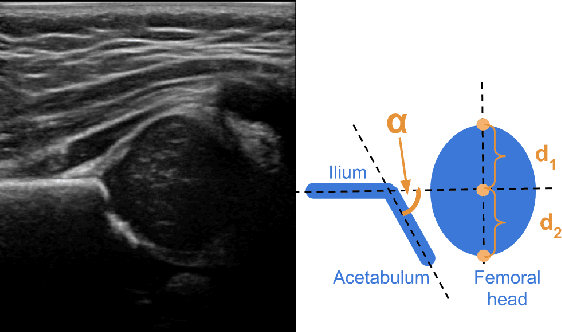
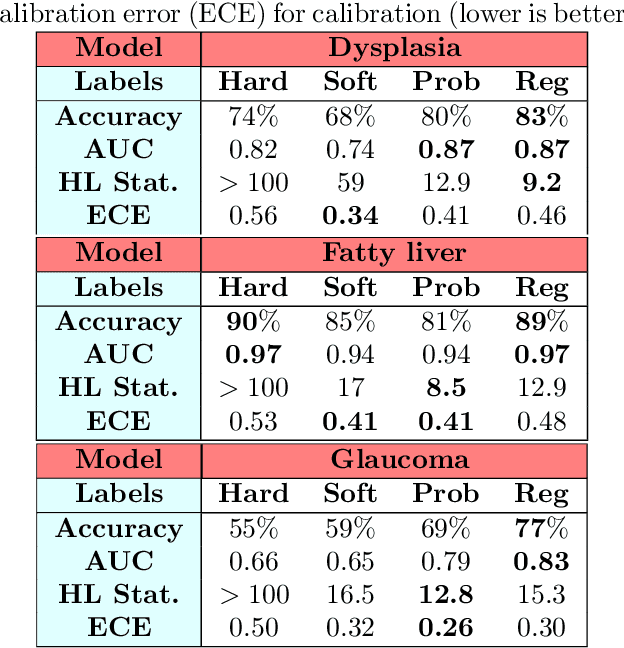
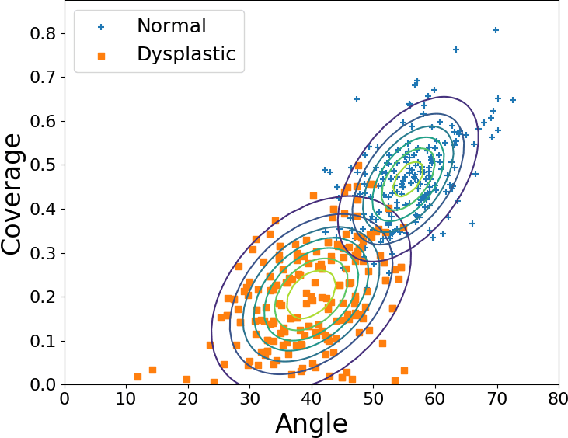

Abstract:Deep learning approaches often require huge datasets to achieve good generalization. This complicates its use in tasks like image-based medical diagnosis, where the small training datasets are usually insufficient to learn appropriate data representations. For such sensitive tasks it is also important to provide the confidence in the predictions. Here, we propose a way to learn and use probabilistic labels to train accurate and calibrated deep networks from relatively small datasets. We observe gains of up to 22% in the accuracy of models trained with these labels, as compared with traditional approaches, in three classification tasks: diagnosis of hip dysplasia, fatty liver, and glaucoma. The outputs of models trained with probabilistic labels are calibrated, allowing the interpretation of its predictions as proper probabilities. We anticipate this approach will apply to other tasks where few training instances are available and expert knowledge can be encoded as probabilities.
A systematic review on the role of artificial intelligence in sonographic diagnosis of thyroid cancer: Past, present and future
Jun 10, 2020


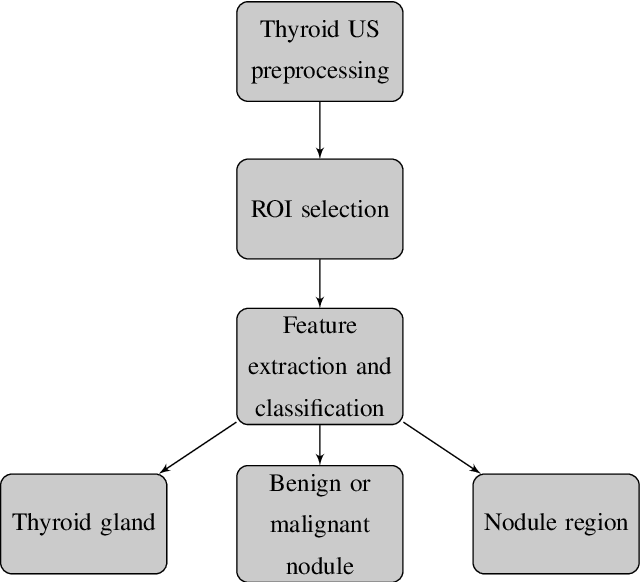
Abstract:Thyroid cancer is common worldwide, with a rapid increase in prevalence across North America in recent years. While most patients present with palpable nodules through physical examination, a large number of small and medium-sized nodules are detected by ultrasound examination. Suspicious nodules are then sent for biopsy through fine needle aspiration. Since biopsies are invasive and sometimes inconclusive, various research groups have tried to develop computer-aided diagnosis systems. Earlier approaches along these lines relied on clinically relevant features that were manually identified by radiologists. With the recent success of artificial intelligence (AI), various new methods are being developed to identify these features in thyroid ultrasound automatically. In this paper, we present a systematic review of state-of-the-art on AI application in sonographic diagnosis of thyroid cancer. This review follows a methodology-based classification of the different techniques available for thyroid cancer diagnosis. With more than 50 papers included in this review, we reflect on the trends and challenges of the field of sonographic diagnosis of thyroid malignancies and potential of computer-aided diagnosis to increase the impact of ultrasound applications on the future of thyroid cancer diagnosis. Machine learning will continue to play a fundamental role in the development of future thyroid cancer diagnosis frameworks.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge