Abhi Lad
Biomedical image analysis competitions: The state of current participation practice
Dec 16, 2022Abstract:The number of international benchmarking competitions is steadily increasing in various fields of machine learning (ML) research and practice. So far, however, little is known about the common practice as well as bottlenecks faced by the community in tackling the research questions posed. To shed light on the status quo of algorithm development in the specific field of biomedical imaging analysis, we designed an international survey that was issued to all participants of challenges conducted in conjunction with the IEEE ISBI 2021 and MICCAI 2021 conferences (80 competitions in total). The survey covered participants' expertise and working environments, their chosen strategies, as well as algorithm characteristics. A median of 72% challenge participants took part in the survey. According to our results, knowledge exchange was the primary incentive (70%) for participation, while the reception of prize money played only a minor role (16%). While a median of 80 working hours was spent on method development, a large portion of participants stated that they did not have enough time for method development (32%). 25% perceived the infrastructure to be a bottleneck. Overall, 94% of all solutions were deep learning-based. Of these, 84% were based on standard architectures. 43% of the respondents reported that the data samples (e.g., images) were too large to be processed at once. This was most commonly addressed by patch-based training (69%), downsampling (37%), and solving 3D analysis tasks as a series of 2D tasks. K-fold cross-validation on the training set was performed by only 37% of the participants and only 50% of the participants performed ensembling based on multiple identical models (61%) or heterogeneous models (39%). 48% of the respondents applied postprocessing steps.
Towards A Device-Independent Deep Learning Approach for the Automated Segmentation of Sonographic Fetal Brain Structures: A Multi-Center and Multi-Device Validation
Feb 28, 2022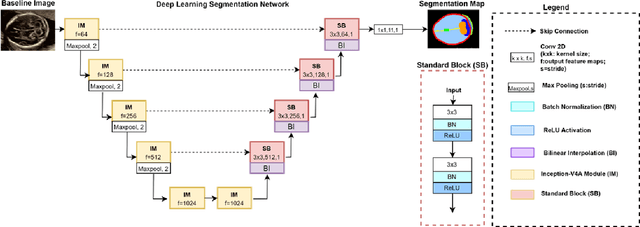
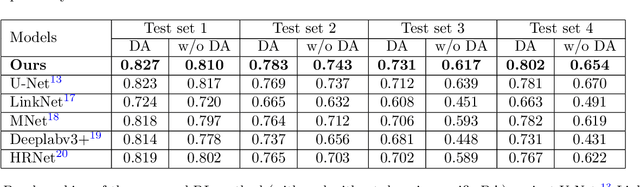
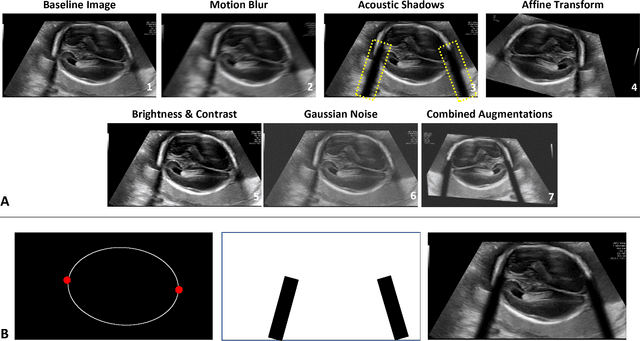
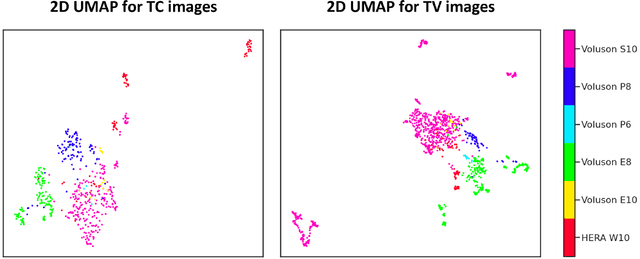
Abstract:Quality assessment of prenatal ultrasonography is essential for the screening of fetal central nervous system (CNS) anomalies. The interpretation of fetal brain structures is highly subjective, expertise-driven, and requires years of training experience, limiting quality prenatal care for all pregnant mothers. With recent advancement in Artificial Intelligence (AI), specifically deep learning (DL), assistance in precise anatomy identification through semantic segmentation essential for the reliable assessment of growth and neurodevelopment, and detection of structural abnormalities have been proposed. However, existing works only identify certain structures (e.g., cavum septum pellucidum, lateral ventricles, cerebellum) from either of the axial views (transventricular, transcerebellar), limiting the scope for a thorough anatomical assessment as per practice guidelines necessary for the screening of CNS anomalies. Further, existing works do not analyze the generalizability of these DL algorithms across images from multiple ultrasound devices and centers, thus, limiting their real-world clinical impact. In this study, we propose a DL based segmentation framework for the automated segmentation of 10 key fetal brain structures from 2 axial planes from fetal brain USG images (2D). We developed a custom U-Net variant that uses inceptionv4 block as a feature extractor and leverages custom domain-specific data augmentation. Quantitatively, the mean (10 structures; test sets 1/2/3/4) Dice-coefficients were: 0.827, 0.802, 0.731, 0.783. Irrespective of the USG device/center, the DL segmentations were qualitatively comparable to their manual segmentations. The proposed DL system offered a promising and generalizable performance (multi-centers, multi-device) and also presents evidence in support of device-induced variation in image quality (a challenge to generalizibility) by using UMAP analysis.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge