Unsupervised Tissue Segmentation via Deep Constrained Gaussian Network
Paper and Code
Aug 04, 2022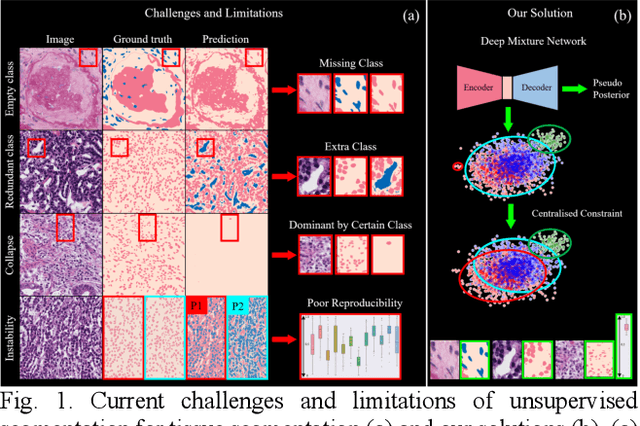
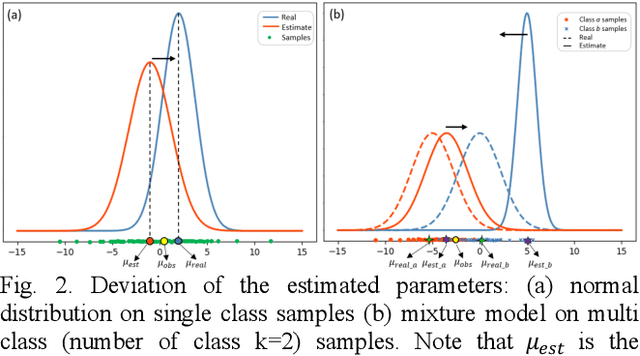
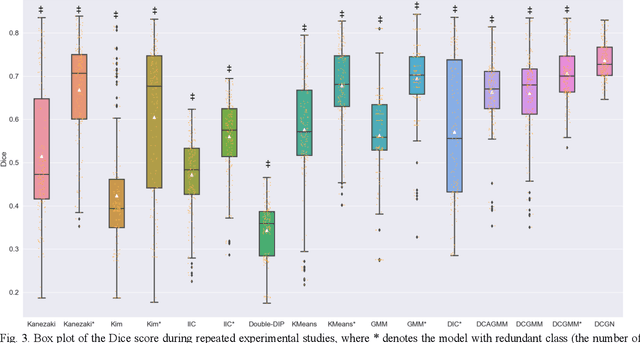
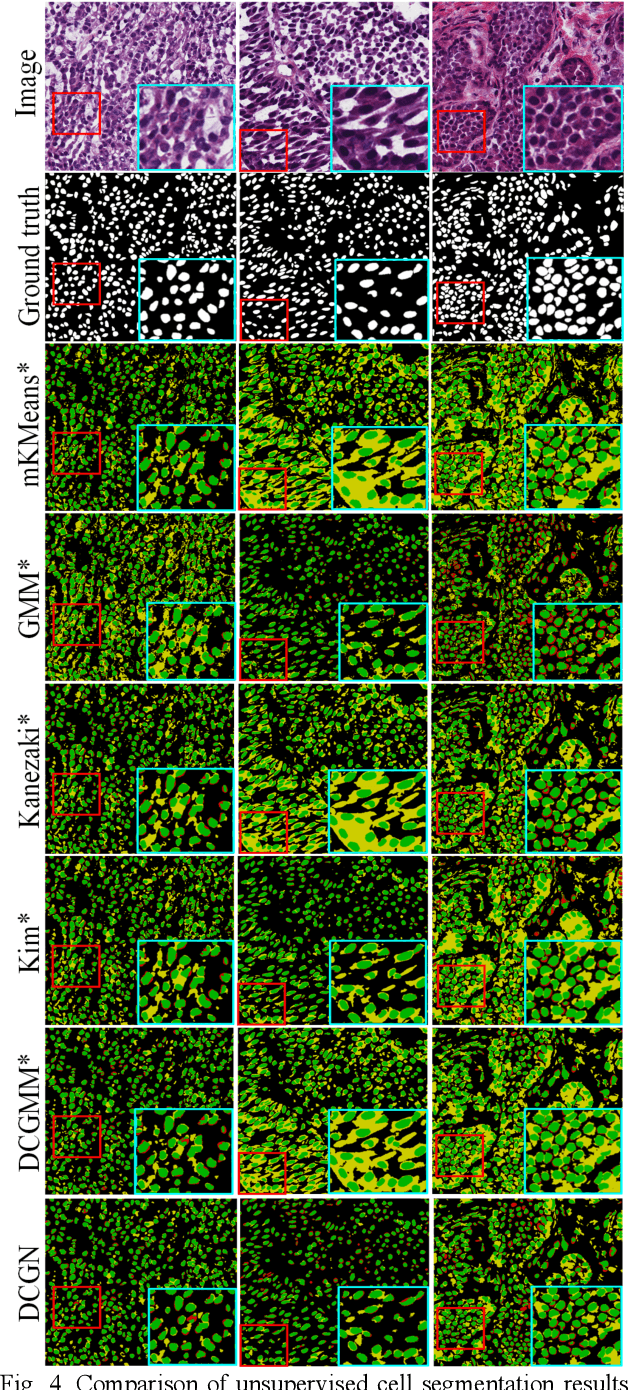
Tissue segmentation is the mainstay of pathological examination, whereas the manual delineation is unduly burdensome. To assist this time-consuming and subjective manual step, researchers have devised methods to automatically segment structures in pathological images. Recently, automated machine and deep learning based methods dominate tissue segmentation research studies. However, most machine and deep learning based approaches are supervised and developed using a large number of training samples, in which the pixelwise annotations are expensive and sometimes can be impossible to obtain. This paper introduces a novel unsupervised learning paradigm by integrating an end-to-end deep mixture model with a constrained indicator to acquire accurate semantic tissue segmentation. This constraint aims to centralise the components of deep mixture models during the calculation of the optimisation function. In so doing, the redundant or empty class issues, which are common in current unsupervised learning methods, can be greatly reduced. By validation on both public and in-house datasets, the proposed deep constrained Gaussian network achieves significantly (Wilcoxon signed-rank test) better performance (with the average Dice scores of 0.737 and 0.735, respectively) on tissue segmentation with improved stability and robustness, compared to other existing unsupervised segmentation approaches. Furthermore, the proposed method presents a similar performance (p-value > 0.05) compared to the fully supervised U-Net.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge