Dual-Reference Source-Free Active Domain Adaptation for Nasopharyngeal Carcinoma Tumor Segmentation across Multiple Hospitals
Paper and Code
Sep 23, 2023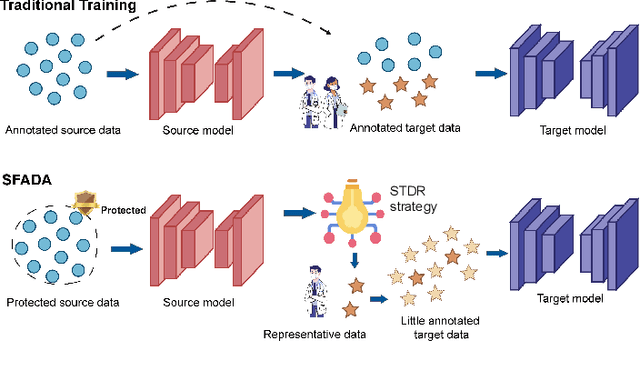
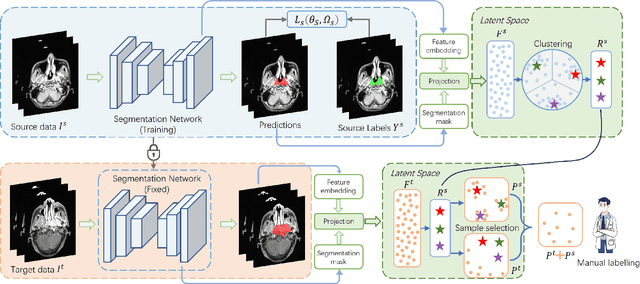
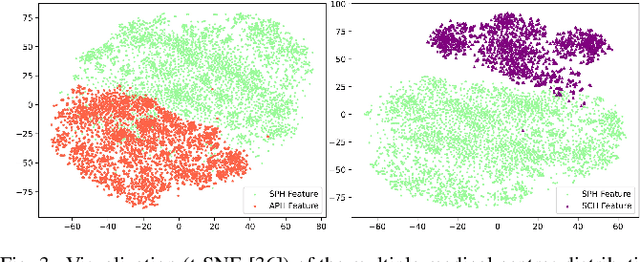
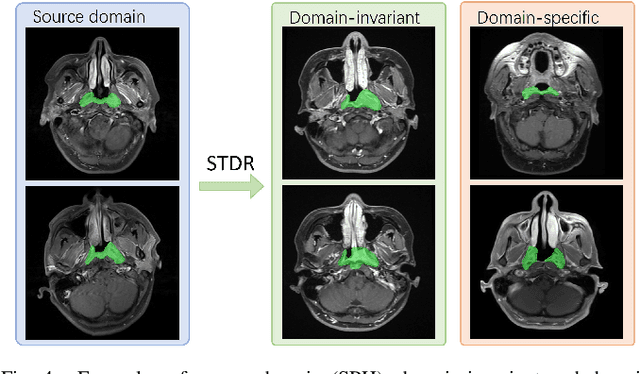
Nasopharyngeal carcinoma (NPC) is a prevalent and clinically significant malignancy that predominantly impacts the head and neck area. Precise delineation of the Gross Tumor Volume (GTV) plays a pivotal role in ensuring effective radiotherapy for NPC. Despite recent methods that have achieved promising results on GTV segmentation, they are still limited by lacking carefully-annotated data and hard-to-access data from multiple hospitals in clinical practice. Although some unsupervised domain adaptation (UDA) has been proposed to alleviate this problem, unconditionally mapping the distribution distorts the underlying structural information, leading to inferior performance. To address this challenge, we devise a novel Sourece-Free Active Domain Adaptation (SFADA) framework to facilitate domain adaptation for the GTV segmentation task. Specifically, we design a dual reference strategy to select domain-invariant and domain-specific representative samples from a specific target domain for annotation and model fine-tuning without relying on source-domain data. Our approach not only ensures data privacy but also reduces the workload for oncologists as it just requires annotating a few representative samples from the target domain and does not need to access the source data. We collect a large-scale clinical dataset comprising 1057 NPC patients from five hospitals to validate our approach. Experimental results show that our method outperforms the UDA methods and achieves comparable results to the fully supervised upper bound, even with few annotations, highlighting the significant medical utility of our approach. In addition, there is no public dataset about multi-center NPC segmentation, we will release code and dataset for future research.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge