Zhinuo
Jenny
Towards Enabling Cardiac Digital Twins of Myocardial Infarction Using Deep Computational Models for Inverse Inference
Jul 13, 2023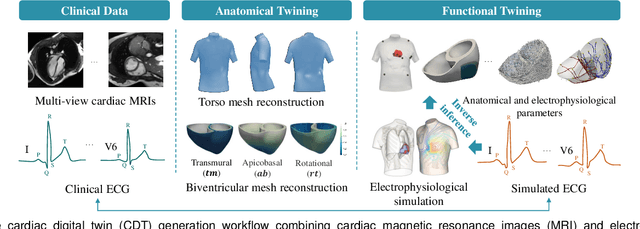
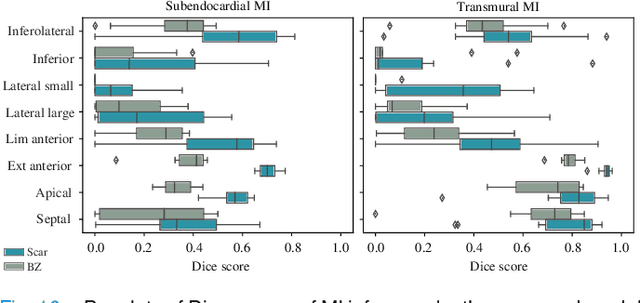
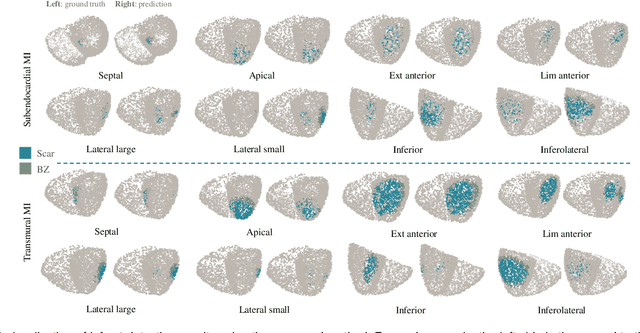
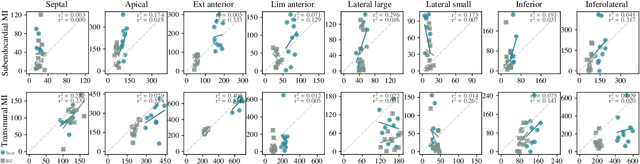
Abstract:Myocardial infarction (MI) demands precise and swift diagnosis. Cardiac digital twins (CDTs) have the potential to offer individualized evaluation of cardiac function in a non-invasive manner, making them a promising approach for personalized diagnosis and treatment planning of MI. The inference of accurate myocardial tissue properties is crucial in creating a reliable CDT platform, and particularly in the context of studying MI. In this work, we investigate the feasibility of inferring myocardial tissue properties from the electrocardiogram (ECG), focusing on the development of a comprehensive CDT platform specifically designed for MI. The platform integrates multi-modal data, such as cardiac MRI and ECG, to enhance the accuracy and reliability of the inferred tissue properties. We perform a sensitivity analysis based on computer simulations, systematically exploring the effects of infarct location, size, degree of transmurality, and electrical activity alteration on the simulated QRS complex of ECG, to establish the limits of the approach. We subsequently propose a deep computational model to infer infarct location and distribution from the simulated QRS. The in silico experimental results show that our model can effectively capture the complex relationships between the QRS signals and the corresponding infarct regions, with promising potential for clinical application in the future. The code will be released publicly once the manuscript is accepted for publication.
Influence of Myocardial Infarction on QRS Properties: A Simulation Study
Apr 21, 2023



Abstract:The interplay between structural and electrical changes in the heart after myocardial infarction (MI) plays a key role in the initiation and maintenance of arrhythmia. The anatomical and electrophysiological properties of scar, border zone, and normal myocardium modify the electrocardiographic morphology, which is routinely analysed in clinical settings. However, the influence of various MI properties on the QRS is not intuitively predictable.In this work, we have systematically investigated the effects of 17 post-MI scenarios, varying the location, size, transmural extent, and conductive level of scarring and border zone area, on the forward-calculated QRS. Additionally, we have compared the contributions of different QRS score criteria for quantifying post-MI pathophysiology.The propagation of electrical activity in the ventricles is simulated via a Eikonal model on a unified coordinate system.The analysis has been performed on 49 subjects, and the results imply that the QRS is capable of identifying MI, suggesting the feasibility of inversely reconstructing infarct regions from QRS.There exist sensitivity variations of different QRS criteria for identifying 17 MI scenarios, which is informative for solving the inverse problem.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge