Yutian Zhong
Performance of Medical Image Fusion in High-level Analysis Tasks: A Mutual Enhancement Framework for Unaligned PAT and MRI Image Fusion
Jul 04, 2024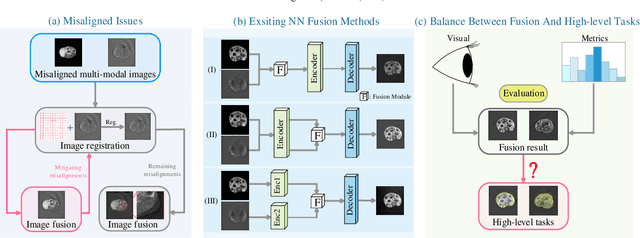
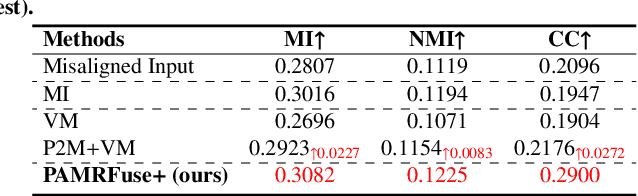
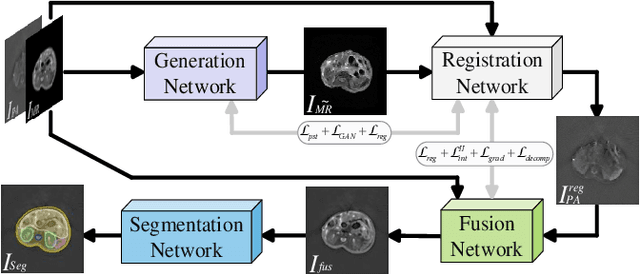
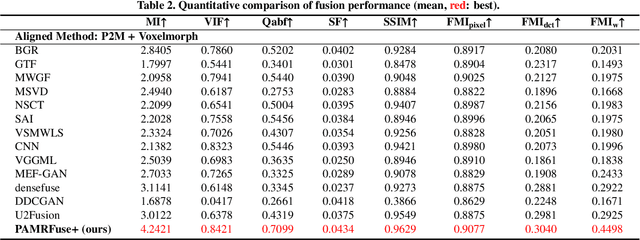
Abstract:Photoacoustic tomography (PAT) offers optical contrast, whereas magnetic resonance imaging (MRI) excels in imaging soft tissue and organ anatomy. The fusion of PAT with MRI holds promising application prospects due to their complementary advantages. Existing image fusion have made considerable progress in pre-registered images, yet spatial deformations are difficult to avoid in medical imaging scenarios. More importantly, current algorithms focus on visual quality and statistical metrics, thus overlooking the requirements of high-level tasks. To address these challenges, we proposes a unsupervised fusion model, termed PAMRFuse+, which integrates image generation and registration. Specifically, a cross-modal style transfer network is introduced to simplify cross-modal registration to single-modal registration. Subsequently, a multi-level registration network is employed to predict displacement vector fields. Furthermore, a dual-branch feature decomposition fusion network is proposed to address the challenges of cross-modal feature modeling and decomposition by integrating modality-specific and modality-shared features. PAMRFuse+ achieves satisfactory results in registering and fusing unaligned PAT-MRI datasets. Moreover, for the first time, we evaluate the performance of medical image fusion with contour segmentation and multi-organ instance segmentation. Extensive experimental demonstrations reveal the advantages of PAMRFuse+ in improving the performance of medical image analysis tasks.
Spiral Scanning and Self-Supervised Image Reconstruction Enable Ultra-Sparse Sampling Multispectral Photoacoustic Tomography
Apr 10, 2024



Abstract:Multispectral photoacoustic tomography (PAT) is an imaging modality that utilizes the photoacoustic effect to achieve non-invasive and high-contrast imaging of internal tissues. However, the hardware cost and computational demand of a multispectral PAT system consisting of up to thousands of detectors are huge. To address this challenge, we propose an ultra-sparse spiral sampling strategy for multispectral PAT, which we named U3S-PAT. Our strategy employs a sparse ring-shaped transducer that, when switching excitation wavelengths, simultaneously rotates and translates. This creates a spiral scanning pattern with multispectral angle-interlaced sampling. To solve the highly ill-conditioned image reconstruction problem, we propose a self-supervised learning method that is able to introduce structural information shared during spiral scanning. We simulate the proposed U3S-PAT method on a commercial PAT system and conduct in vivo animal experiments to verify its performance. The results show that even with a sparse sampling rate as low as 1/30, our U3S-PAT strategy achieves similar reconstruction and spectral unmixing accuracy as non-spiral dense sampling. Given its ability to dramatically reduce the time required for three-dimensional multispectral scanning, our U3S-PAT strategy has the potential to perform volumetric molecular imaging of dynamic biological activities.
Deep learning acceleration of iterative model-based light fluence correction for photoacoustic tomography
Dec 08, 2023Abstract:Photoacoustic tomography (PAT) is a promising imaging technique that can visualize the distribution of chromophores within biological tissue. However, the accuracy of PAT imaging is compromised by light fluence (LF), which hinders the quantification of light absorbers. Currently, model-based iterative methods are used for LF correction, but they require significant computational resources due to repeated LF estimation based on differential light transport models. To improve LF correction efficiency, we propose to use Fourier neural operator (FNO), a neural network specially designed for solving differential equations, to learn the forward projection of light transport in PAT. Trained using paired finite-element-based LF simulation data, our FNO model replaces the traditional computational heavy LF estimator during iterative correction, such that the correction procedure is significantly accelerated. Simulation and experimental results demonstrate that our method achieves comparable LF correction quality to traditional iterative methods while reducing the correction time by over 30 times.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge