Yunzhe Qiu
Neural network-based on-chip spectroscopy using a scalable plasmonic encoder
Dec 01, 2020

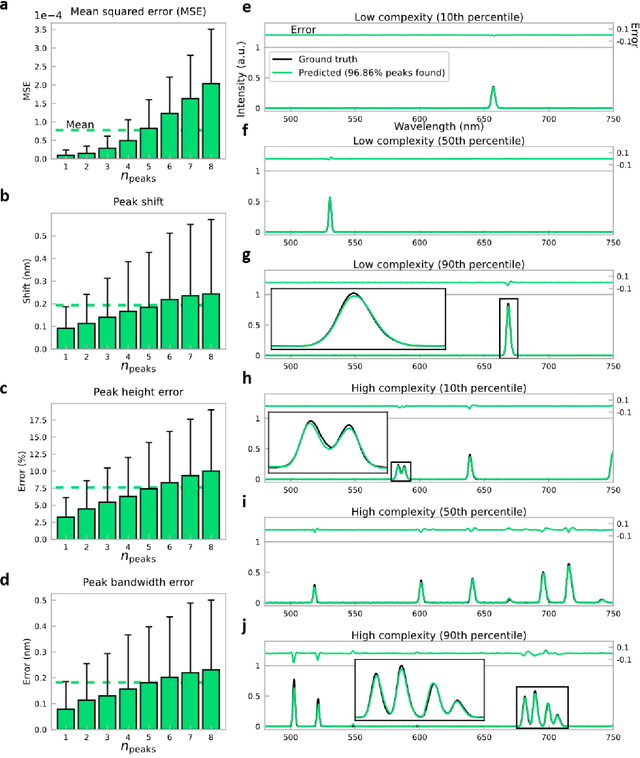
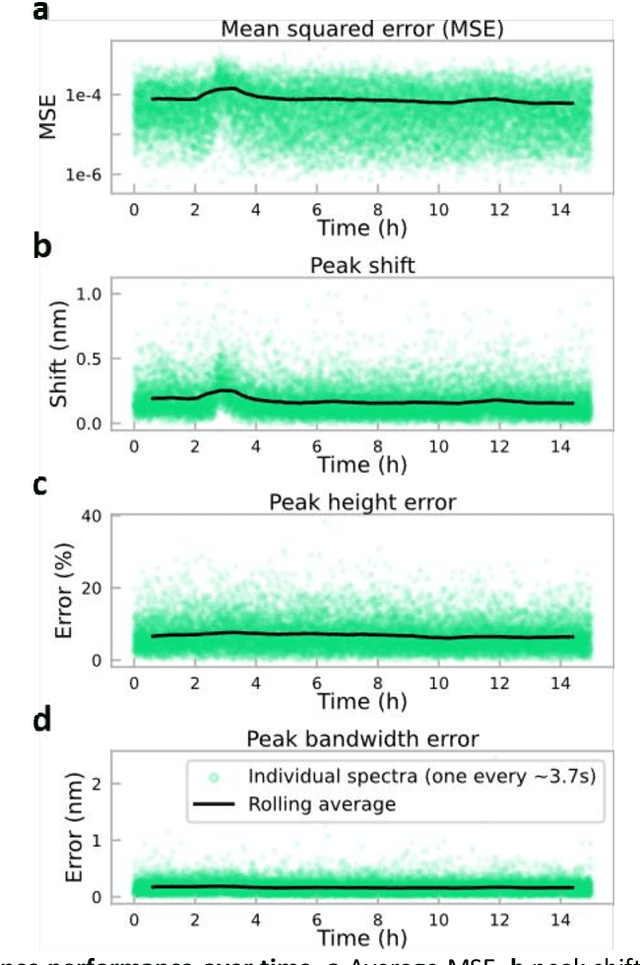
Abstract:Conventional spectrometers are limited by trade-offs set by size, cost, signal-to-noise ratio (SNR), and spectral resolution. Here, we demonstrate a deep learning-based spectral reconstruction framework, using a compact and low-cost on-chip sensing scheme that is not constrained by the design trade-offs inherent to grating-based spectroscopy. The system employs a plasmonic spectral encoder chip containing 252 different tiles of nanohole arrays fabricated using a scalable and low-cost imprint lithography method, where each tile has a unique geometry and, thus, a unique optical transmission spectrum. The illumination spectrum of interest directly impinges upon the plasmonic encoder, and a CMOS image sensor captures the transmitted light, without any lenses, gratings, or other optical components in between, making the entire hardware highly compact, light-weight and field-portable. A trained neural network then reconstructs the unknown spectrum using the transmitted intensity information from the spectral encoder in a feed-forward and non-iterative manner. Benefiting from the parallelization of neural networks, the average inference time per spectrum is ~28 microseconds, which is orders of magnitude faster compared to other computational spectroscopy approaches. When blindly tested on unseen new spectra (N = 14,648) with varying complexity, our deep-learning based system identified 96.86% of the spectral peaks with an average peak localization error, bandwidth error, and height error of 0.19 nm, 0.18 nm, and 7.60%, respectively. This system is also highly tolerant to fabrication defects that may arise during the imprint lithography process, which further makes it ideal for applications that demand cost-effective, field-portable and sensitive high-resolution spectroscopy tools.
Early-detection and classification of live bacteria using time-lapse coherent imaging and deep learning
Jan 29, 2020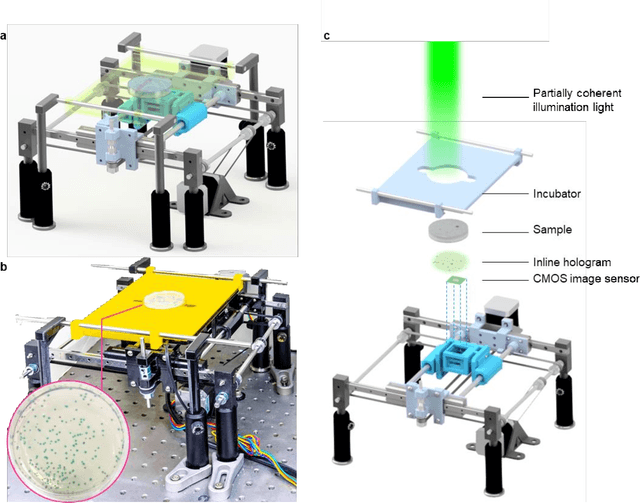
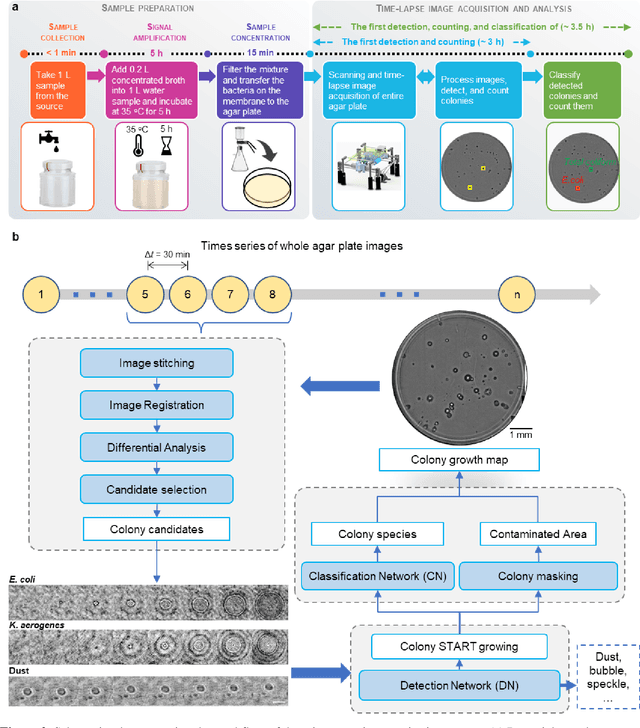
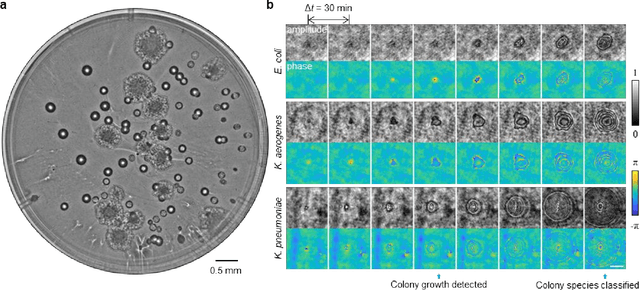
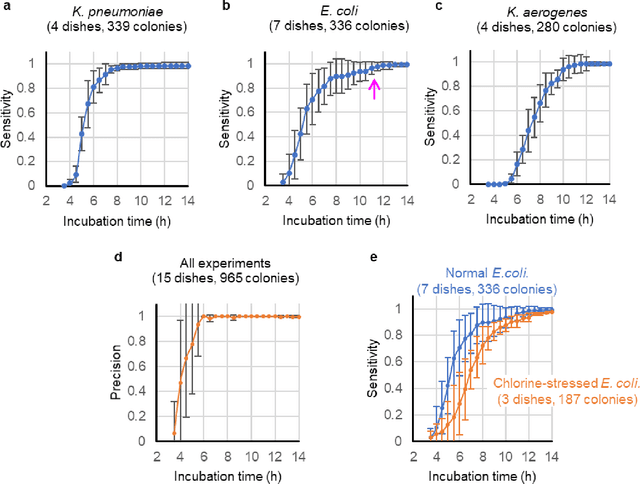
Abstract:We present a computational live bacteria detection system that periodically captures coherent microscopy images of bacterial growth inside a 60 mm diameter agar-plate and analyzes these time-lapsed holograms using deep neural networks for rapid detection of bacterial growth and classification of the corresponding species. The performance of our system was demonstrated by rapid detection of Escherichia coli and total coliform bacteria (i.e., Klebsiella aerogenes and Klebsiella pneumoniae subsp. pneumoniae) in water samples. These results were confirmed against gold-standard culture-based results, shortening the detection time of bacterial growth by >12 h as compared to the Environmental Protection Agency (EPA)-approved analytical methods. Our experiments further confirmed that this method successfully detects 90% of bacterial colonies within 7-10 h (and >95% within 12 h) with a precision of 99.2-100%, and correctly identifies their species in 7.6-12 h with 80% accuracy. Using pre-incubation of samples in growth media, our system achieved a limit of detection (LOD) of ~1 colony forming unit (CFU)/L within 9 h of total test time. This computational bacteria detection and classification platform is highly cost-effective (~$0.6 per test) and high-throughput with a scanning speed of 24 cm2/min over the entire plate surface, making it highly suitable for integration with the existing analytical methods currently used for bacteria detection on agar plates. Powered by deep learning, this automated and cost-effective live bacteria detection platform can be transformative for a wide range of applications in microbiology by significantly reducing the detection time, also automating the identification of colonies, without labeling or the need for an expert.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge