Xavier Rafael-Palou
Eurecat Centre Tecnològic de Catalunya, eHealth Unit, Barcelona, Spain, BCN MedTech, Dept. of Information and Communication Technologies, Universitat Pompeu Fabra, Barcelona, Spain
Agent-Based Output Drift Detection for Breast Cancer Response Prediction in a Multisite Clinical Decision Support System
Dec 20, 2025Abstract:Modern clinical decision support systems can concurrently serve multiple, independent medical imaging institutions, but their predictive performance may degrade across sites due to variations in patient populations, imaging hardware, and acquisition protocols. Continuous surveillance of predictive model outputs offers a safe and reliable approach for identifying such distributional shifts without ground truth labels. However, most existing methods rely on centralized monitoring of aggregated predictions, overlooking site-specific drift dynamics. We propose an agent-based framework for detecting drift and assessing its severity in multisite clinical AI systems. To evaluate its effectiveness, we simulate a multi-center environment for output-based drift detection, assigning each site a drift monitoring agent that performs batch-wise comparisons of model outputs against a reference distribution. We analyse several multi-center monitoring schemes, that differ in how the reference is obtained (site-specific, global, production-only and adaptive), alongside a centralized baseline. Results on real-world breast cancer imaging data using a pathological complete response prediction model shows that all multi-center schemes outperform centralized monitoring, with F1-score improvements up to 10.3% in drift detection. In the absence of site-specific references, the adaptive scheme performs best, with F1-scores of 74.3% for drift detection and 83.7% for drift severity classification. These findings suggest that adaptive, site-aware agent-based drift monitoring can enhance reliability of multisite clinical decision support systems.
An Uncertainty-aware Hierarchical Probabilistic Network for Early Prediction, Quantification and Segmentation of Pulmonary Tumour Growth
Apr 18, 2021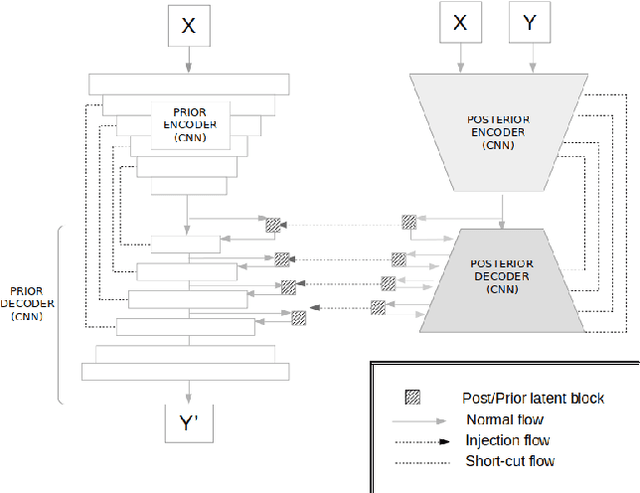
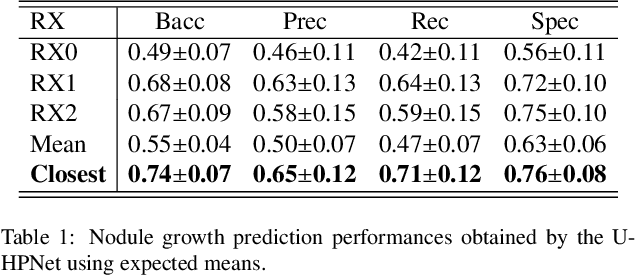
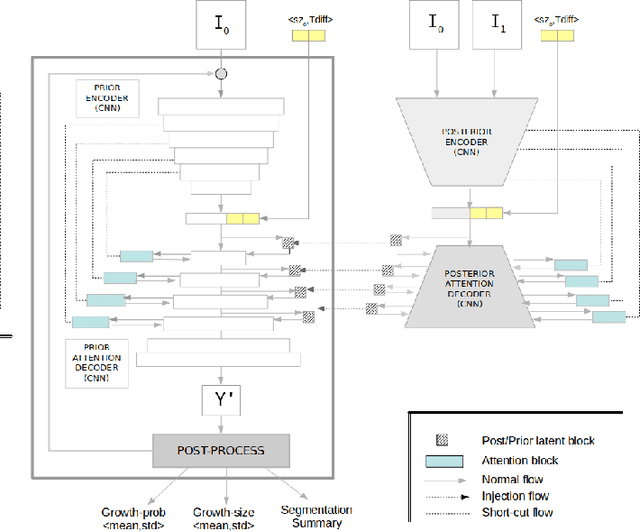
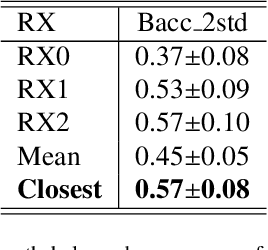
Abstract:Early detection and quantification of tumour growth would help clinicians to prescribe more accurate treatments and provide better surgical planning. However, the multifactorial and heterogeneous nature of lung tumour progression hampers identification of growth patterns. In this study, we present a novel method based on a deep hierarchical generative and probabilistic framework that, according to radiological guidelines, predicts tumour growth, quantifies its size and provides a semantic appearance of the future nodule. Unlike previous deterministic solutions, the generative characteristic of our approach also allows us to estimate the uncertainty in the predictions, especially important for complex and doubtful cases. Results of evaluating this method on an independent test set reported a tumour growth balanced accuracy of 74%, a tumour growth size MAE of 1.77 mm and a tumour segmentation Dice score of 78%. These surpassed the performances of equivalent deterministic and alternative generative solutions (i.e. probabilistic U-Net, Bayesian test dropout and Pix2Pix GAN) confirming the suitability of our approach.
Detection, growth quantification and malignancy prediction of pulmonary nodules using deep convolutional networks in follow-up CT scans
Mar 26, 2021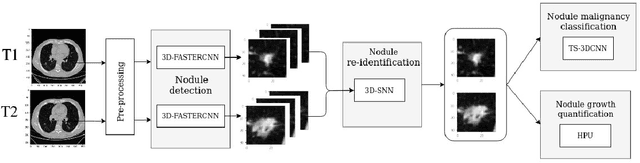
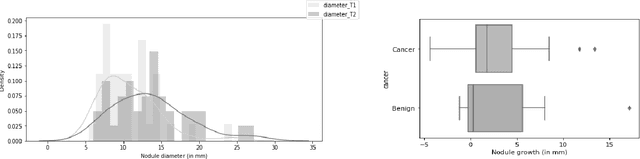
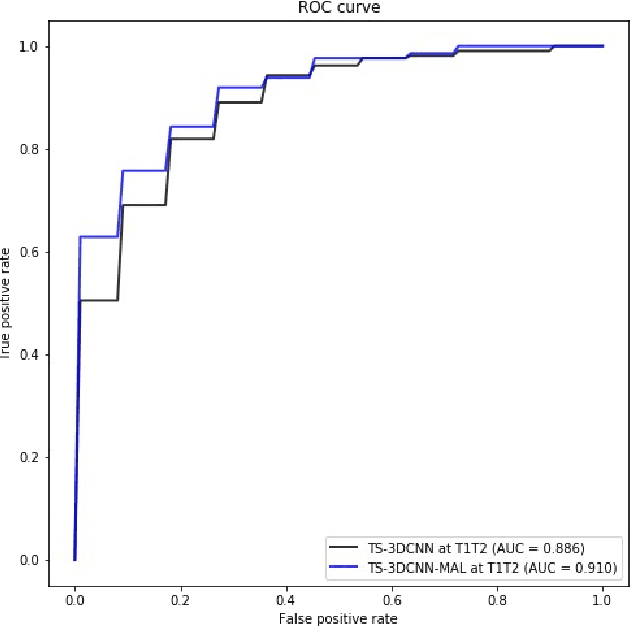
Abstract:We address the problem of supporting radiologists in the longitudinal management of lung cancer. Therefore, we proposed a deep learning pipeline, composed of four stages that completely automatized from the detection of nodules to the classification of cancer, through the detection of growth in the nodules. In addition, the pipeline integrated a novel approach for nodule growth detection, which relied on a recent hierarchical probabilistic U-Net adapted to report uncertainty estimates. Also, a second novel method was introduced for lung cancer nodule classification, integrating into a two stream 3D-CNN network the estimated nodule malignancy probabilities derived from a pretrained nodule malignancy network. The pipeline was evaluated in a longitudinal cohort and reported comparable performances to the state of art.
Pulmonary Nodule Malignancy Classification Using its Temporal Evolution with Two-Stream 3D Convolutional Neural Networks
May 22, 2020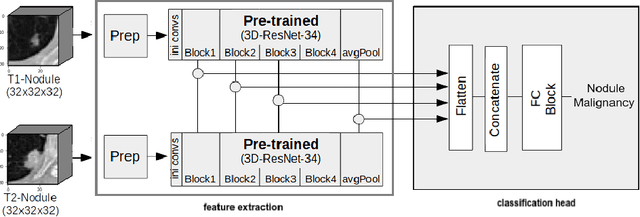
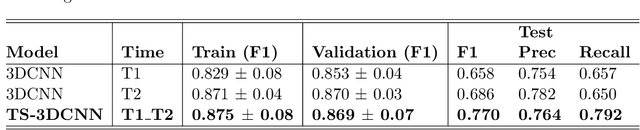
Abstract:Nodule malignancy assessment is a complex, time-consuming and error-prone task. Current clinical practice requires measuring changes in size and density of the nodule at different time-points. State of the art solutions rely on 3D convolutional neural networks built on pulmonary nodules obtained from single CT scan per patient. In this work, we propose a two-stream 3D convolutional neural network that predicts malignancy by jointly analyzing two pulmonary nodule volumes from the same patient taken at different time-points. Best results achieve 77% of F1-score in test with an increment of 9% and 12% of F1-score with respect to the same network trained with images from a single time-point.
Comparative Analysis of Predictive Methods for Early Assessment of Compliance with Continuous Positive Airway Pressure Therapy
Dec 27, 2019



Abstract:Patients suffering from obstructive sleep apnea are mainly treated with continuous positive airway pressure (CPAP). Good compliance with this therapy is broadly accepted as more than 4h of CPAP average use nightly. Although it is a highly effective treatment, compliance with this therapy is problematic to achieve with serious consequences for the patients' health. Previous works already reported factors significantly related to compliance with the therapy. However, further research is still required to support clinicians to early anticipate patients' therapy compliance. This work intends to take a further step in this direction by building compliance classifiers with CPAP therapy at three different moments of the patient follow-up (i.e. before the therapy starts and at months 1 and 3 after the baseline). Results of the clinical trial confirmed that month 3 was the time-point with the most accurate classifier reaching an f1-score of 87% and 84% in cross-validation and test. At month 1, performances were almost as high as in month 3 with 82% and 84% of f1-score. At baseline, where no information about patients' CPAP use was given yet, the best classifier achieved 73% and 76% of f1-score in cross-validation and test set respectively. Subsequent analyses carried out with the best classifiers of each time point revealed that certain baseline factors (i.e. headaches, psychological symptoms, arterial hypertension and EuroQol visual analogue scale) were closely related to the prediction of compliance independently of the time-point. In addition, among the variables taken only during the follow-up of the patients, Epworth and the average nighttime hours were the most important to predict compliance with CPAP.
* 22 pages, 11 figures
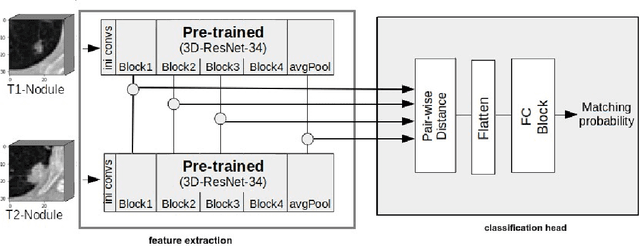
Re-Identification and Growth Detection of Pulmonary Nodules without Image Registration Using 3D Siamese Neural Networks
Dec 22, 2019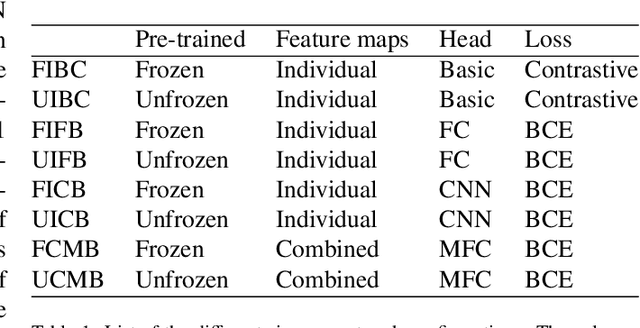
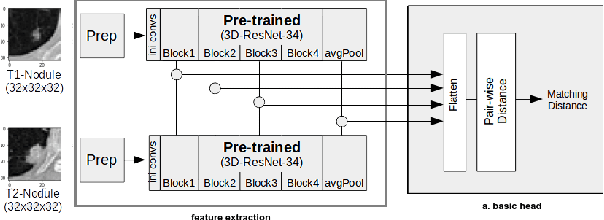
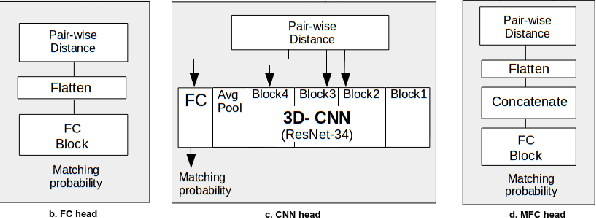
Abstract:Lung cancer follow-up is a complex, error prone, and time consuming task for clinical radiologists. Several lung CT scan images taken at different time points of a given patient need to be individually inspected, looking for possible cancerogenous nodules. Radiologists mainly focus their attention in nodule size, density, and growth to assess the existence of malignancy. In this study, we present a novel method based on a 3D siamese neural network, for the re-identification of nodules in a pair of CT scans of the same patient without the need for image registration. The network was integrated into a two-stage automatic pipeline to detect, match, and predict nodule growth given pairs of CT scans. Results on an independent test set reported a nodule detection sensitivity of 94.7%, an accuracy for temporal nodule matching of 88.8%, and a sensitivity of 92.0% with a precision of 88.4% for nodule growth detection.
Integration of Convolutional Neural Networks for Pulmonary Nodule Malignancy Assessment in a Lung Cancer Classification Pipeline
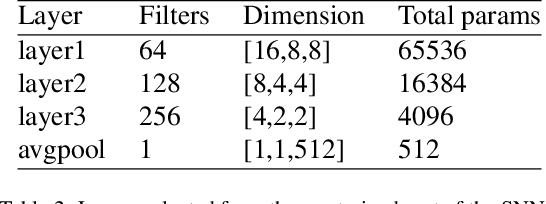
Dec 18, 2019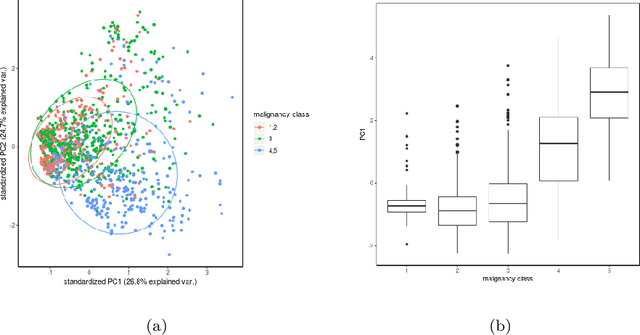
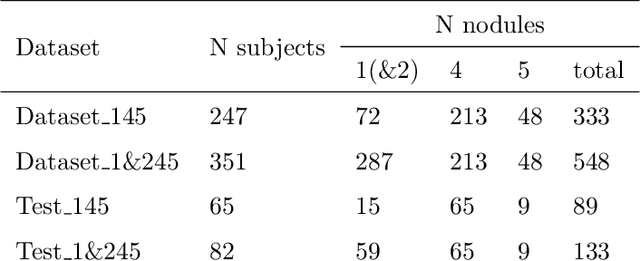
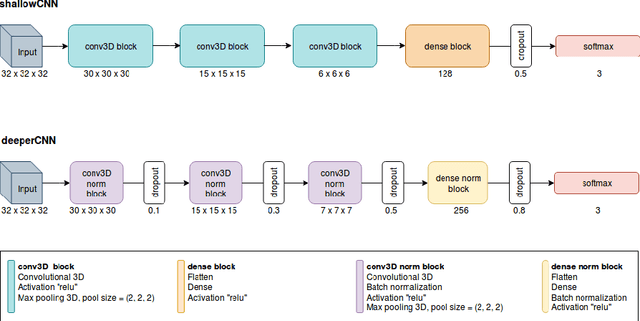
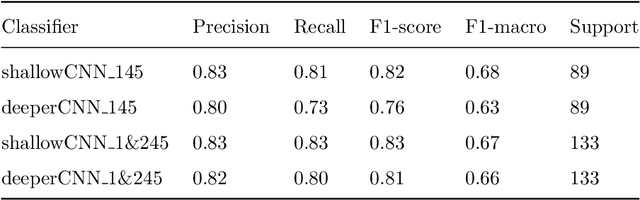
Abstract:The early identification of malignant pulmonary nodules is critical for better lung cancer prognosis and less invasive chemo or radio therapies. Nodule malignancy assessment done by radiologists is extremely useful for planning a preventive intervention but is, unfortunately, a complex, time-consuming and error-prone task. This explains the lack of large datasets containing radiologists malignancy characterization of nodules. In this article, we propose to assess nodule malignancy through 3D convolutional neural networks and to integrate it in an automated end-to-end existing pipeline of lung cancer detection. For training and testing purposes we used independent subsets of the LIDC dataset. Adding the probabilities of nodules malignity in a baseline lung cancer pipeline improved its F1-weighted score by 14.7%, whereas integrating the malignancy model itself using transfer learning outperformed the baseline prediction by 11.8% of F1-weighted score. Despite the limited size of the lung cancer datasets, integrating predictive models of nodule malignancy improves prediction of lung cancer.
* 26 pages, 5 figures
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge