Wolfgang Fruehwirt
Bayesian deep neural networks for low-cost neurophysiological markers of Alzheimer's disease severity
Dec 13, 2018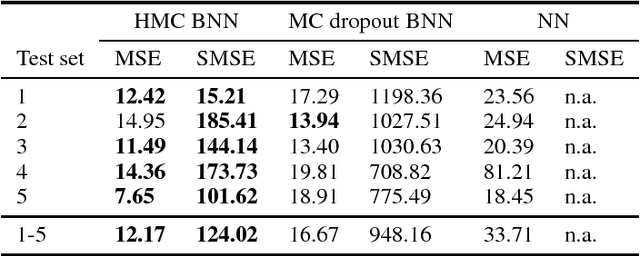
Abstract:As societies around the world are ageing, the number of Alzheimer's disease (AD) patients is rapidly increasing. To date, no low-cost, non-invasive biomarkers have been established to advance the objectivization of AD diagnosis and progression assessment. Here, we utilize Bayesian neural networks to develop a multivariate predictor for AD severity using a wide range of quantitative EEG (QEEG) markers. The Bayesian treatment of neural networks both automatically controls model complexity and provides a predictive distribution over the target function, giving uncertainty bounds for our regression task. It is therefore well suited to clinical neuroscience, where data sets are typically sparse and practitioners require a precise assessment of the predictive uncertainty. We use data of one of the largest prospective AD EEG trials ever conducted to demonstrate the potential of Bayesian deep learning in this domain, while comparing two distinct Bayesian neural network approaches, i.e., Monte Carlo dropout and Hamiltonian Monte Carlo.
Sensor Selection and Random Field Reconstruction for Robust and Cost-effective Heterogeneous Weather Sensor Networks for the Developing World
Nov 23, 2017

Abstract:We address the two fundamental problems of spatial field reconstruction and sensor selection in heterogeneous sensor networks: (i) how to efficiently perform spatial field reconstruction based on measurements obtained simultaneously from networks with both high and low quality sensors; and (ii) how to perform query based sensor set selection with predictive MSE performance guarantee. For the first problem, we developed a low complexity algorithm based on the spatial best linear unbiased estimator (S-BLUE). Next, building on the S-BLUE, we address the second problem, and develop an efficient algorithm for query based sensor set selection with performance guarantee. Our algorithm is based on the Cross Entropy method which solves the combinatorial optimization problem in an efficient manner.
Riemannian tangent space mapping and elastic net regularization for cost-effective EEG markers of brain atrophy in Alzheimer's disease
Nov 22, 2017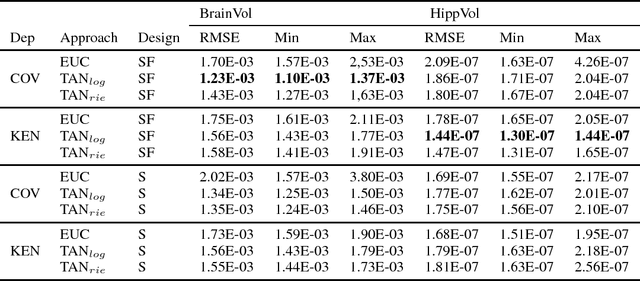
Abstract:The diagnosis of Alzheimer's disease (AD) in routine clinical practice is most commonly based on subjective clinical interpretations. Quantitative electroencephalography (QEEG) measures have been shown to reflect neurodegenerative processes in AD and might qualify as affordable and thereby widely available markers to facilitate the objectivization of AD assessment. Here, we present a novel framework combining Riemannian tangent space mapping and elastic net regression for the development of brain atrophy markers. While most AD QEEG studies are based on small sample sizes and psychological test scores as outcome measures, here we train and test our models using data of one of the largest prospective EEG AD trials ever conducted, including MRI biomarkers of brain atrophy.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge