Vejay Vakharia
Brain Lesion Synthesis via Progressive Adversarial Variational Auto-Encoder
Aug 05, 2022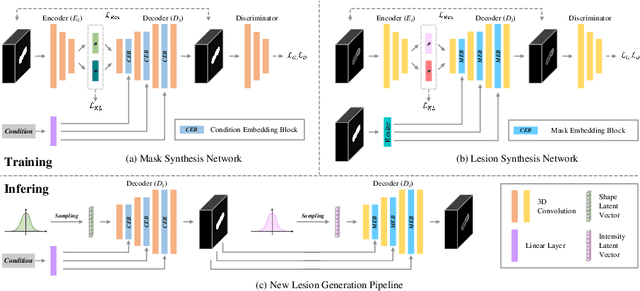
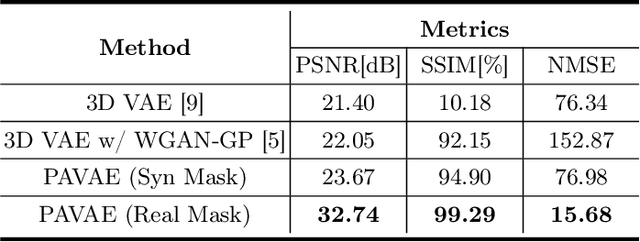
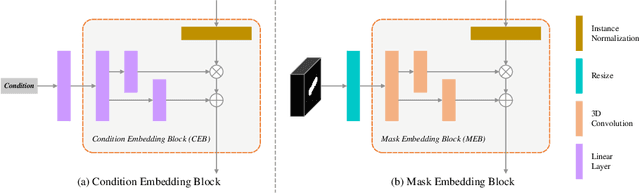
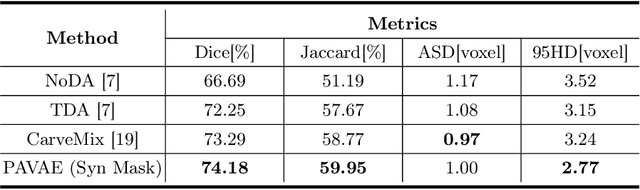
Abstract:Laser interstitial thermal therapy (LITT) is a novel minimally invasive treatment that is used to ablate intracranial structures to treat mesial temporal lobe epilepsy (MTLE). Region of interest (ROI) segmentation before and after LITT would enable automated lesion quantification to objectively assess treatment efficacy. Deep learning techniques, such as convolutional neural networks (CNNs) are state-of-the-art solutions for ROI segmentation, but require large amounts of annotated data during the training. However, collecting large datasets from emerging treatments such as LITT is impractical. In this paper, we propose a progressive brain lesion synthesis framework (PAVAE) to expand both the quantity and diversity of the training dataset. Concretely, our framework consists of two sequential networks: a mask synthesis network and a mask-guided lesion synthesis network. To better employ extrinsic information to provide additional supervision during network training, we design a condition embedding block (CEB) and a mask embedding block (MEB) to encode inherent conditions of masks to the feature space. Finally, a segmentation network is trained using raw and synthetic lesion images to evaluate the effectiveness of the proposed framework. Experimental results show that our method can achieve realistic synthetic results and boost the performance of down-stream segmentation tasks above traditional data augmentation techniques.
Using convolution neural networks to learn enhanced fiber orientation distribution models from commercially available diffusion magnetic resonance imaging
Aug 12, 2020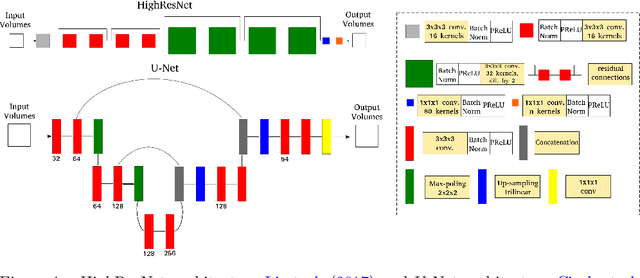
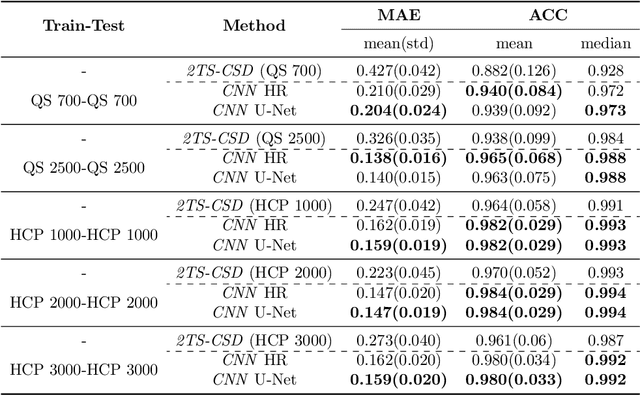
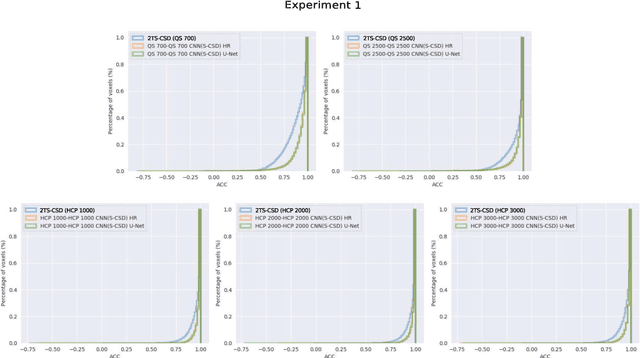
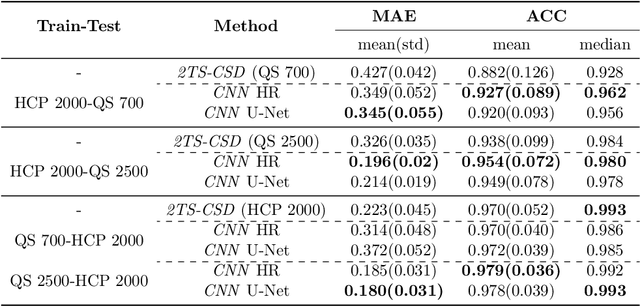
Abstract:Accurate local fiber orientation distribution (FOD) modeling based on diffusion magnetic resonance imaging (dMRI) capable of resolving complex fiber configurations benefit from specific acquisition protocols that impose a high number of gradient directions (b-vecs), a high maximum b-value (b-vals) and multiple b-values (multi-shell). However, acquisition time is limited in a clinical setting and commercial scanners may not provide robust state-of-the-art dMRI sequences. Therefore, dMRI is often acquired as single-shell (SS) (single b-value). Here, we learn improved FODs for commercially acquired dMRI. We evaluate the use of 3D convolutional neural networks (CNNs) to regress multi-shell FOS representations from single-shell representations, using the spherical harmonics basis obtained from constrained spherical deconvolution (CSD) to model FODs. We use U-Net and HighResNet 3D CNN architectures and data from the publicly available Human Connectome Dataset and a dataset acquired at National Hospital For Neurology and Neurosurgery Queen Square. We evaluate how well the CNN models can resolve local fiber orientation 1) when training and testing on datasets with same dMRI acquisition protocol; 2) when testing on dataset with a different dMRI acquisition protocol than used training the CNN models; and 3) when testing on datasets with a fewer number dMRI gradient directions than used training the CNN models. Our approach may enable robust CSD model estimation on dMRI acquisition protocols which are single shell and with a few gradient directions, reducing acquisition times, and thus, facilitating translation to time-limited clinical environments.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge