Teja Krishna Cherukuri
DiA-gnostic VLVAE: Disentangled Alignment-Constrained Vision Language Variational AutoEncoder for Robust Radiology Reporting with Missing Modalities
Nov 08, 2025Abstract:The integration of medical images with clinical context is essential for generating accurate and clinically interpretable radiology reports. However, current automated methods often rely on resource-heavy Large Language Models (LLMs) or static knowledge graphs and struggle with two fundamental challenges in real-world clinical data: (1) missing modalities, such as incomplete clinical context , and (2) feature entanglement, where mixed modality-specific and shared information leads to suboptimal fusion and clinically unfaithful hallucinated findings. To address these challenges, we propose the DiA-gnostic VLVAE, which achieves robust radiology reporting through Disentangled Alignment. Our framework is designed to be resilient to missing modalities by disentangling shared and modality-specific features using a Mixture-of-Experts (MoE) based Vision-Language Variational Autoencoder (VLVAE). A constrained optimization objective enforces orthogonality and alignment between these latent representations to prevent suboptimal fusion. A compact LLaMA-X decoder then uses these disentangled representations to generate reports efficiently. On the IU X-Ray and MIMIC-CXR datasets, DiA has achieved competetive BLEU@4 scores of 0.266 and 0.134, respectively. Experimental results show that the proposed method significantly outperforms state-of-the-art models.
Dynamic Contextual Attention Network: Transforming Spatial Representations into Adaptive Insights for Endoscopic Polyp Diagnosis
Apr 28, 2025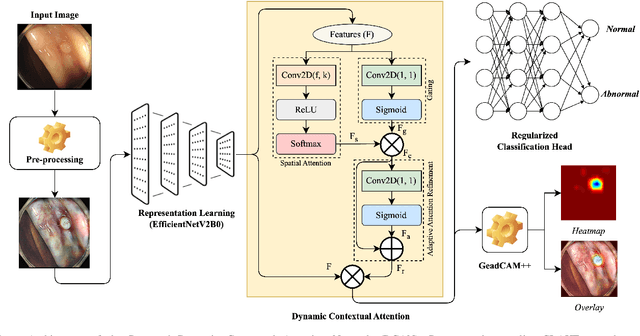
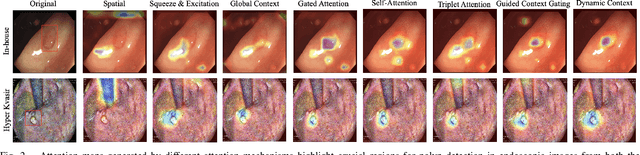
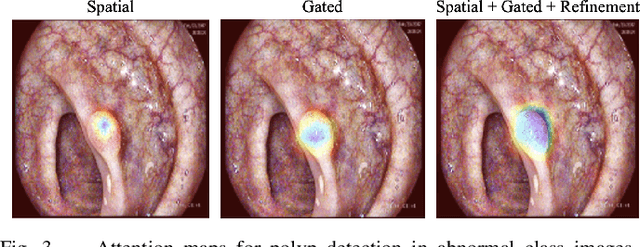
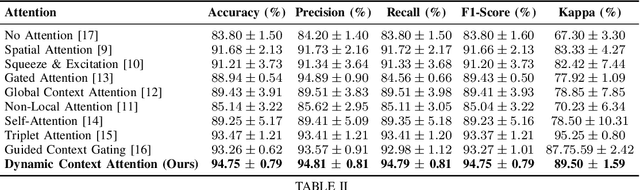
Abstract:Colorectal polyps are key indicators for early detection of colorectal cancer. However, traditional endoscopic imaging often struggles with accurate polyp localization and lacks comprehensive contextual awareness, which can limit the explainability of diagnoses. To address these issues, we propose the Dynamic Contextual Attention Network (DCAN). This novel approach transforms spatial representations into adaptive contextual insights, using an attention mechanism that enhances focus on critical polyp regions without explicit localization modules. By integrating contextual awareness into the classification process, DCAN improves decision interpretability and overall diagnostic performance. This advancement in imaging could lead to more reliable colorectal cancer detection, enabling better patient outcomes.
GCS-M3VLT: Guided Context Self-Attention based Multi-modal Medical Vision Language Transformer for Retinal Image Captioning
Dec 23, 2024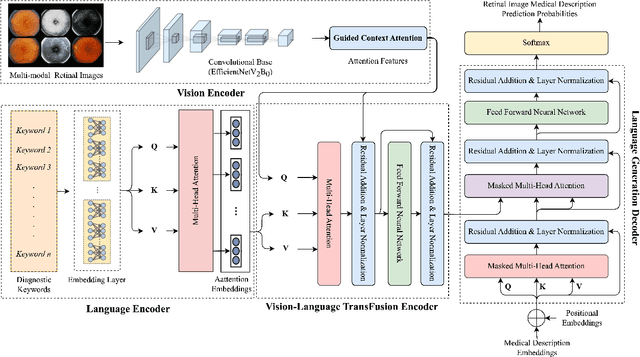
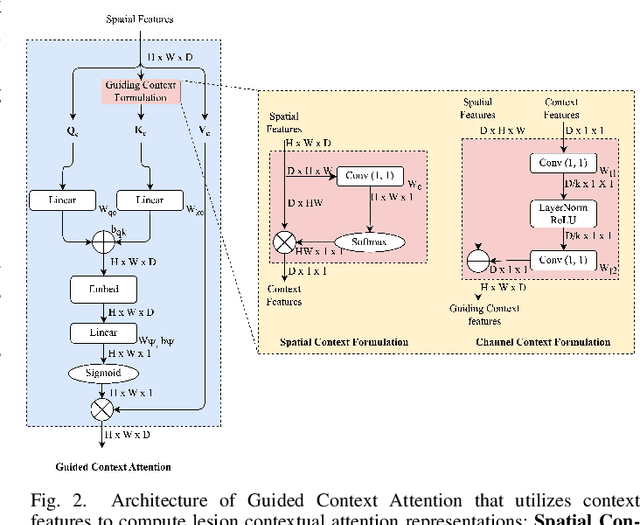
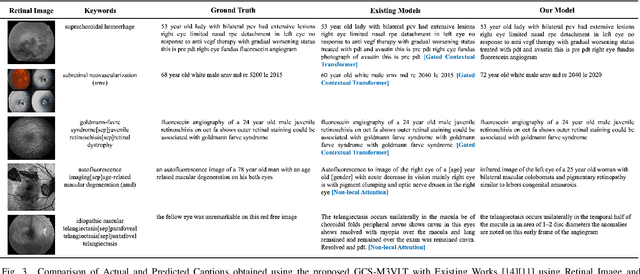
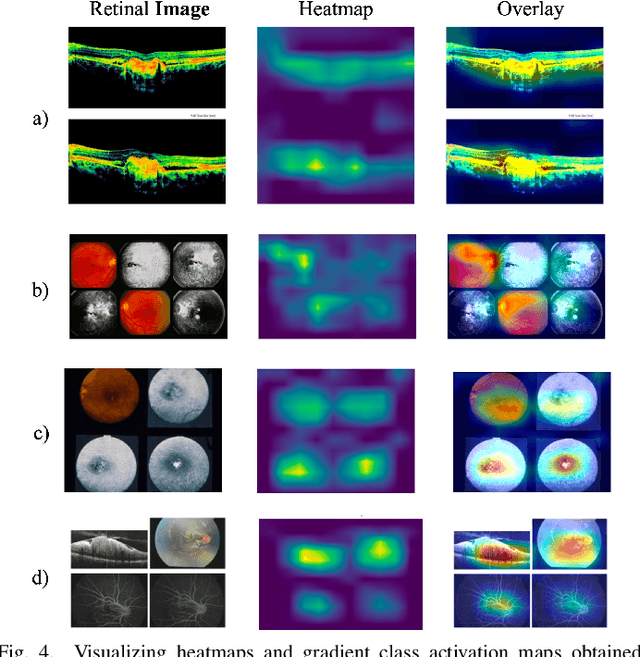
Abstract:Retinal image analysis is crucial for diagnosing and treating eye diseases, yet generating accurate medical reports from images remains challenging due to variability in image quality and pathology, especially with limited labeled data. Previous Transformer-based models struggled to integrate visual and textual information under limited supervision. In response, we propose a novel vision-language model for retinal image captioning that combines visual and textual features through a guided context self-attention mechanism. This approach captures both intricate details and the global clinical context, even in data-scarce scenarios. Extensive experiments on the DeepEyeNet dataset demonstrate a 0.023 BLEU@4 improvement, along with significant qualitative advancements, highlighting the effectiveness of our model in generating comprehensive medical captions.
Multi-modal Imaging Genomics Transformer: Attentive Integration of Imaging with Genomic Biomarkers for Schizophrenia Classification
Jul 28, 2024


Abstract:Schizophrenia (SZ) is a severe brain disorder marked by diverse cognitive impairments, abnormalities in brain structure, function, and genetic factors. Its complex symptoms and overlap with other psychiatric conditions challenge traditional diagnostic methods, necessitating advanced systems to improve precision. Existing research studies have mostly focused on imaging data, such as structural and functional MRI, for SZ diagnosis. There has been less focus on the integration of genomic features despite their potential in identifying heritable SZ traits. In this study, we introduce a Multi-modal Imaging Genomics Transformer (MIGTrans), that attentively integrates genomics with structural and functional imaging data to capture SZ-related neuroanatomical and connectome abnormalities. MIGTrans demonstrated improved SZ classification performance with an accuracy of 86.05% (+/- 0.02), offering clear interpretations and identifying significant genomic locations and brain morphological/connectivity patterns associated with SZ.
Guided Context Gating: Learning to leverage salient lesions in retinal fundus images
Jun 19, 2024Abstract:Effectively representing medical images, especially retinal images, presents a considerable challenge due to variations in appearance, size, and contextual information of pathological signs called lesions. Precise discrimination of these lesions is crucial for diagnosing vision-threatening issues such as diabetic retinopathy. While visual attention-based neural networks have been introduced to learn spatial context and channel correlations from retinal images, they often fall short in capturing localized lesion context. Addressing this limitation, we propose a novel attention mechanism called Guided Context Gating, an unique approach that integrates Context Formulation, Channel Correlation, and Guided Gating to learn global context, spatial correlations, and localized lesion context. Our qualitative evaluation against existing attention mechanisms emphasize the superiority of Guided Context Gating in terms of explainability. Notably, experiments on the Zenodo-DR-7 dataset reveal a substantial 2.63% accuracy boost over advanced attention mechanisms & an impressive 6.53% improvement over the state-of-the-art Vision Transformer for assessing the severity grade of retinopathy, even with imbalanced and limited training samples for each class.
M3T: Multi-Modal Medical Transformer to bridge Clinical Context with Visual Insights for Retinal Image Medical Description Generation
Jun 19, 2024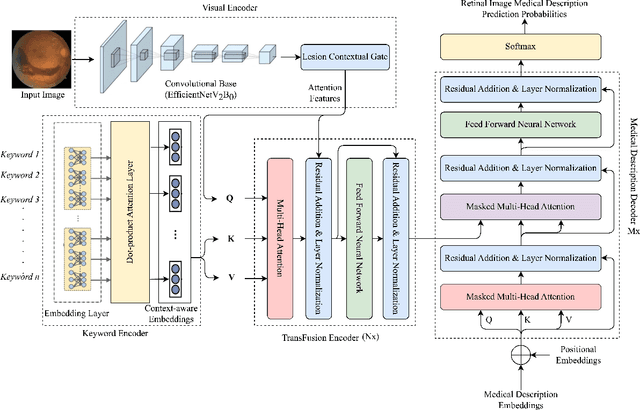
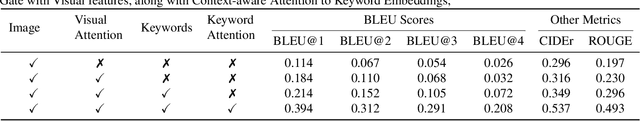
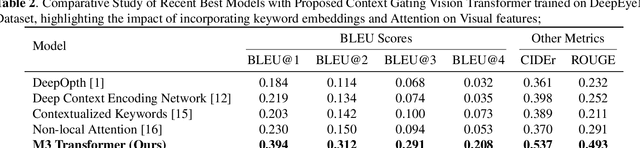
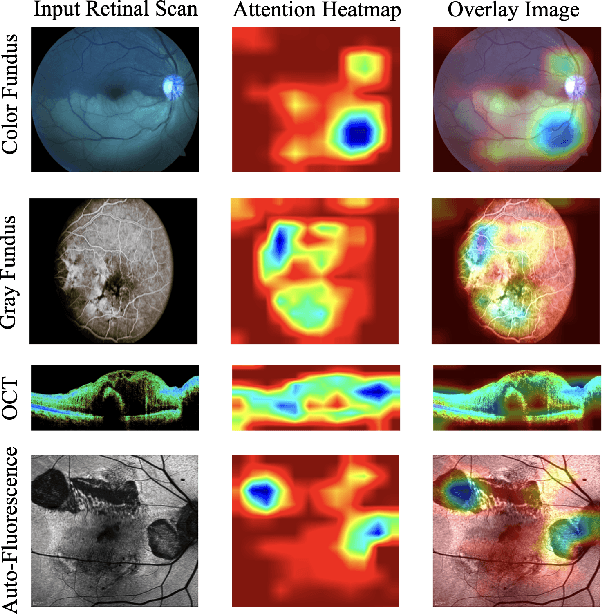
Abstract:Automated retinal image medical description generation is crucial for streamlining medical diagnosis and treatment planning. Existing challenges include the reliance on learned retinal image representations, difficulties in handling multiple imaging modalities, and the lack of clinical context in visual representations. Addressing these issues, we propose the Multi-Modal Medical Transformer (M3T), a novel deep learning architecture that integrates visual representations with diagnostic keywords. Unlike previous studies focusing on specific aspects, our approach efficiently learns contextual information and semantics from both modalities, enabling the generation of precise and coherent medical descriptions for retinal images. Experimental studies on the DeepEyeNet dataset validate the success of M3T in meeting ophthalmologists' standards, demonstrating a substantial 13.5% improvement in BLEU@4 over the best-performing baseline model.
Spatial Sequence Attention Network for Schizophrenia Classification from Structural Brain MR Images
Jun 18, 2024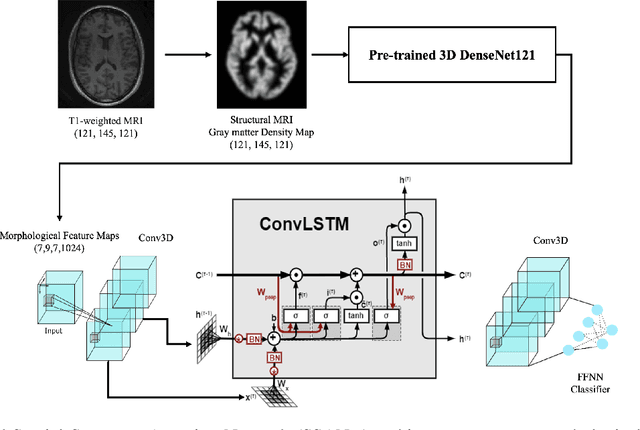
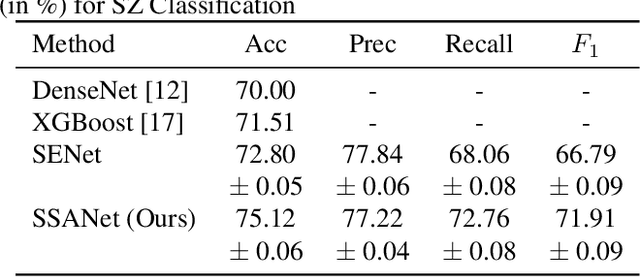
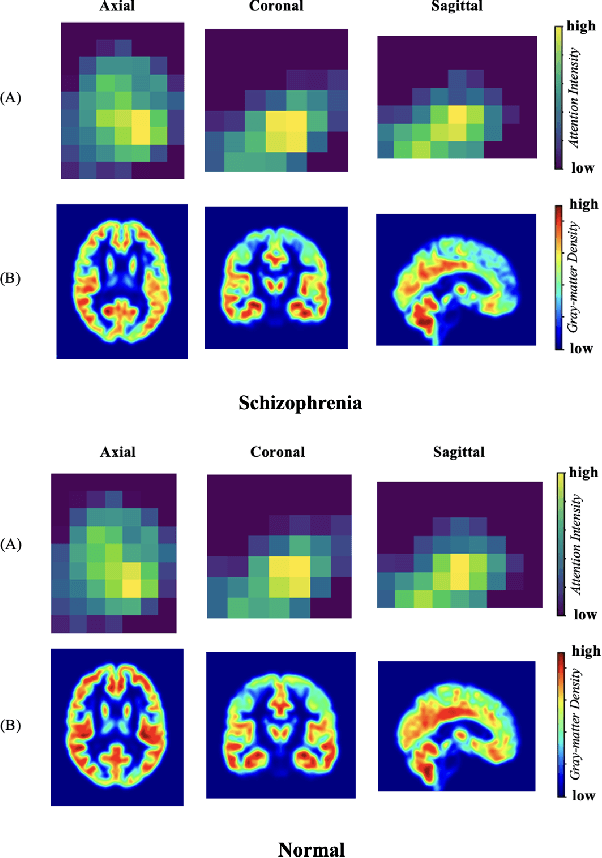
Abstract:Schizophrenia is a debilitating, chronic mental disorder that significantly impacts an individual's cognitive abilities, behavior, and social interactions. It is characterized by subtle morphological changes in the brain, particularly in the gray matter. These changes are often imperceptible through manual observation, demanding an automated approach to diagnosis. This study introduces a deep learning methodology for the classification of individuals with Schizophrenia. We achieve this by implementing a diversified attention mechanism known as Spatial Sequence Attention (SSA) which is designed to extract and emphasize significant feature representations from structural MRI (sMRI). Initially, we employ the transfer learning paradigm by leveraging pre-trained DenseNet to extract initial feature maps from the final convolutional block which contains morphological alterations associated with Schizophrenia. These features are further processed by the proposed SSA to capture and emphasize intricate spatial interactions and relationships across volumes within the brain. Our experimental studies conducted on a clinical dataset have revealed that the proposed attention mechanism outperforms the existing Squeeze & Excitation Network for Schizophrenia classification.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge