Tanvir Hossain
Tackling Oversmoothing in GNN via Graph Sparsification: A Truss-based Approach
Jul 16, 2024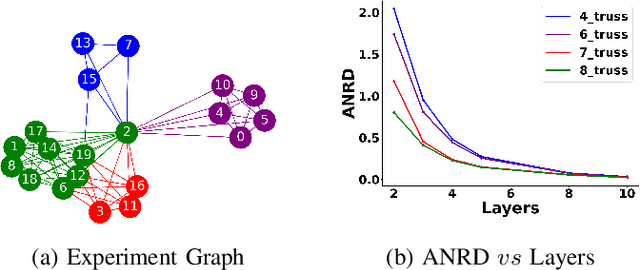
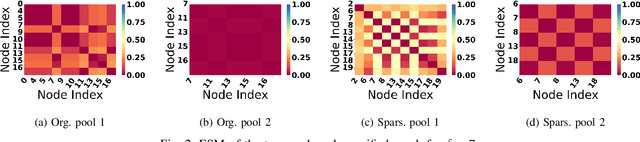
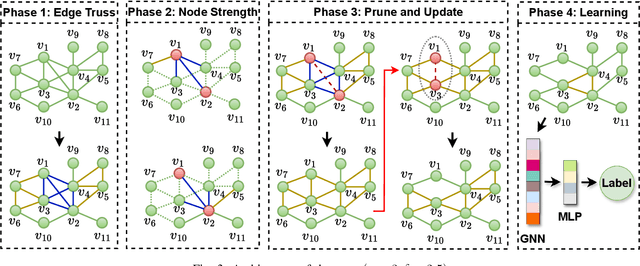
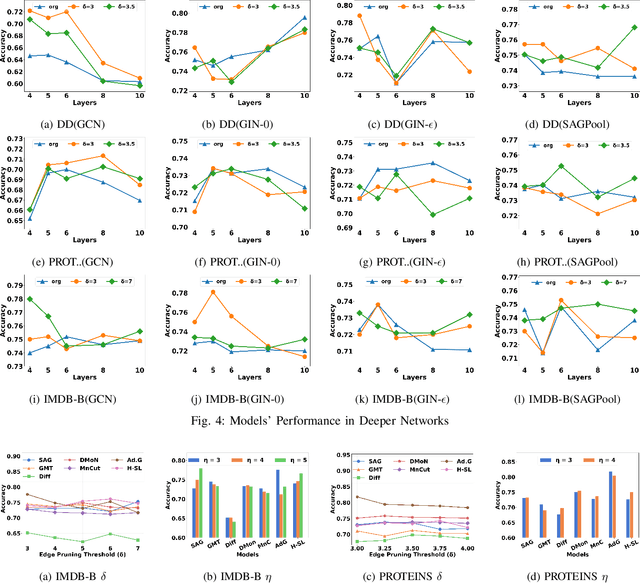
Abstract:Graph Neural Network (GNN) achieves great success for node-level and graph-level tasks via encoding meaningful topological structures of networks in various domains, ranging from social to biological networks. However, repeated aggregation operations lead to excessive mixing of node representations, particularly in dense regions with multiple GNN layers, resulting in nearly indistinguishable embeddings. This phenomenon leads to the oversmoothing problem that hampers downstream graph analytics tasks. To overcome this issue, we propose a novel and flexible truss-based graph sparsification model that prunes edges from dense regions of the graph. Pruning redundant edges in dense regions helps to prevent the aggregation of excessive neighborhood information during hierarchical message passing and pooling in GNN models. We then utilize our sparsification model in the state-of-the-art baseline GNNs and pooling models, such as GIN, SAGPool, GMT, DiffPool, MinCutPool, HGP-SL, DMonPool, and AdamGNN. Extensive experiments on different real-world datasets show that our model significantly improves the performance of the baseline GNN models in the graph classification task.
HeTriNet: Heterogeneous Graph Triplet Attention Network for Drug-Target-Disease Interaction
Nov 30, 2023
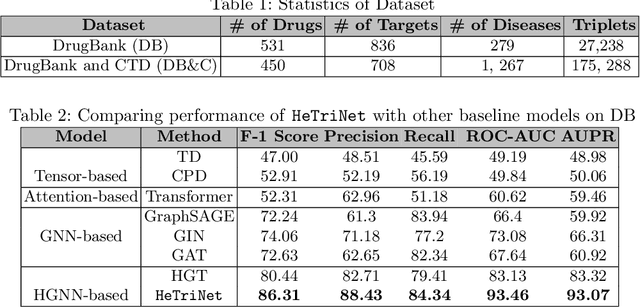
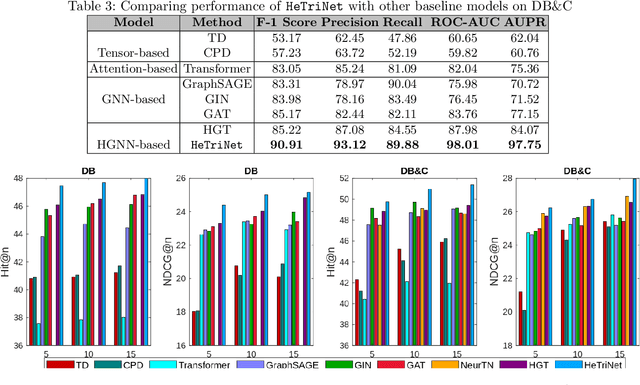

Abstract:Modeling the interactions between drugs, targets, and diseases is paramount in drug discovery and has significant implications for precision medicine and personalized treatments. Current approaches frequently consider drug-target or drug-disease interactions individually, ignoring the interdependencies among all three entities. Within human metabolic systems, drugs interact with protein targets in cells, influencing target activities and subsequently impacting biological pathways to promote healthy functions and treat diseases. Moving beyond binary relationships and exploring tighter triple relationships is essential to understanding drugs' mechanism of action (MoAs). Moreover, identifying the heterogeneity of drugs, targets, and diseases, along with their distinct characteristics, is critical to model these complex interactions appropriately. To address these challenges, we effectively model the interconnectedness of all entities in a heterogeneous graph and develop a novel Heterogeneous Graph Triplet Attention Network (\texttt{HeTriNet}). \texttt{HeTriNet} introduces a novel triplet attention mechanism within this heterogeneous graph structure. Beyond pairwise attention as the importance of an entity for the other one, we define triplet attention to model the importance of pairs for entities in the drug-target-disease triplet prediction problem. Experimental results on real-world datasets show that \texttt{HeTriNet} outperforms several baselines, demonstrating its remarkable proficiency in uncovering novel drug-target-disease relationships.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge