Sina Gholami
Multi-OCT-SelfNet: Integrating Self-Supervised Learning with Multi-Source Data Fusion for Enhanced Multi-Class Retinal Disease Classification
Sep 17, 2024
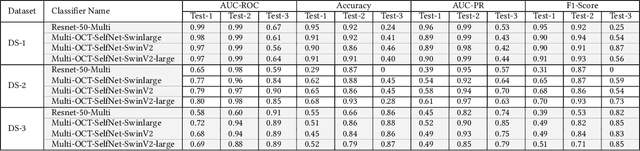
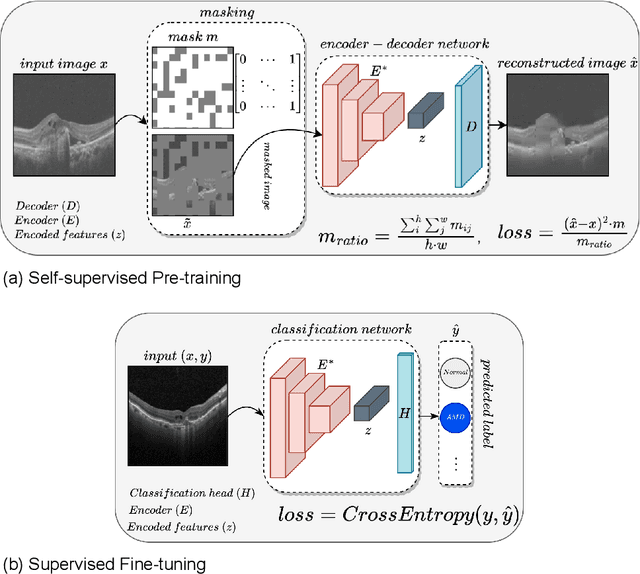
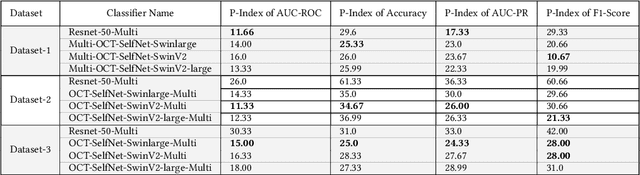
Abstract:In the medical domain, acquiring large datasets poses significant challenges due to privacy concerns. Nonetheless, the development of a robust deep-learning model for retinal disease diagnosis necessitates a substantial dataset for training. The capacity to generalize effectively on smaller datasets remains a persistent challenge. The scarcity of data presents a significant barrier to the practical implementation of scalable medical AI solutions. To address this issue, we've combined a wide range of data sources to improve performance and generalization to new data by giving it a deeper understanding of the data representation from multi-modal datasets and developed a self-supervised framework based on large language models (LLMs), SwinV2 to gain a deeper understanding of multi-modal dataset representations, enhancing the model's ability to extrapolate to new data for the detection of eye diseases using optical coherence tomography (OCT) images. We adopt a two-phase training methodology, self-supervised pre-training, and fine-tuning on a downstream supervised classifier. An ablation study conducted across three datasets employing various encoder backbones, without data fusion, with low data availability setting, and without self-supervised pre-training scenarios, highlights the robustness of our method. Our findings demonstrate consistent performance across these diverse conditions, showcasing superior generalization capabilities compared to the baseline model, ResNet-50.
OCT-SelfNet: A Self-Supervised Framework with Multi-Modal Datasets for Generalized and Robust Retinal Disease Detection
Jan 22, 2024



Abstract:Despite the revolutionary impact of AI and the development of locally trained algorithms, achieving widespread generalized learning from multi-modal data in medical AI remains a significant challenge. This gap hinders the practical deployment of scalable medical AI solutions. Addressing this challenge, our research contributes a self-supervised robust machine learning framework, OCT-SelfNet, for detecting eye diseases using optical coherence tomography (OCT) images. In this work, various data sets from various institutions are combined enabling a more comprehensive range of representation. Our method addresses the issue using a two-phase training approach that combines self-supervised pretraining and supervised fine-tuning with a mask autoencoder based on the SwinV2 backbone by providing a solution for real-world clinical deployment. Extensive experiments on three datasets with different encoder backbones, low data settings, unseen data settings, and the effect of augmentation show that our method outperforms the baseline model, Resnet-50 by consistently attaining AUC-ROC performance surpassing 77% across all tests, whereas the baseline model exceeds 54%. Moreover, in terms of the AUC-PR metric, our proposed method exceeded 42%, showcasing a substantial increase of at least 10% in performance compared to the baseline, which exceeded only 33%. This contributes to our understanding of our approach's potential and emphasizes its usefulness in clinical settings.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge