Siamak Pedrammehr
Enhancing Cognitive Workload Classification Using Integrated LSTM Layers and CNNs for fNIRS Data Analysis
Jul 22, 2024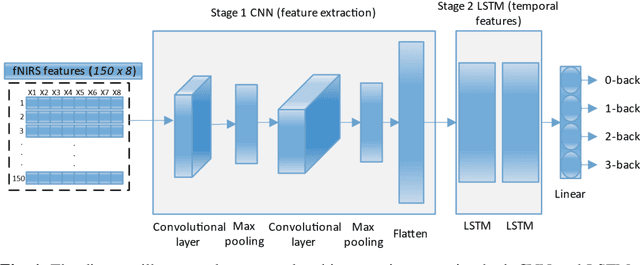

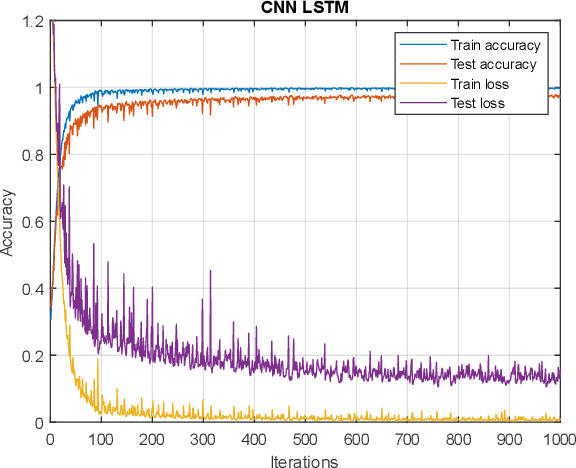

Abstract:Functional near-infrared spectroscopy (fNIRS) is employed as a non-invasive method to monitor functional brain activation by capturing changes in the concentrations of oxygenated haemoglobin (HbO) and deoxygenated haemo-globin (HbR). Various machine learning classification techniques have been utilized to distinguish cognitive states. However, conventional machine learning methods, although simpler to implement, undergo a complex pre-processing phase before network training and demonstrate reduced accuracy due to inadequate data preprocessing. Additionally, previous research in cog-nitive load assessment using fNIRS has predominantly focused on differ-sizeentiating between two levels of mental workload. These studies mainly aim to classify low and high levels of cognitive load or distinguish between easy and difficult tasks. To address these limitations associated with conven-tional methods, this paper conducts a comprehensive exploration of the im-pact of Long Short-Term Memory (LSTM) layers on the effectiveness of Convolutional Neural Networks (CNNs) within deep learning models. This is to address the issues related to spatial features overfitting and lack of tem-poral dependencies in CNN in the previous studies. By integrating LSTM layers, the model can capture temporal dependencies in the fNIRS data, al-lowing for a more comprehensive understanding of cognitive states. The primary objective is to assess how incorporating LSTM layers enhances the performance of CNNs. The experimental results presented in this paper demonstrate that the integration of LSTM layers with Convolutional layers results in an increase in the accuracy of deep learning models from 97.40% to 97.92%.
Advancements in Radiomics and Artificial Intelligence for Thyroid Cancer Diagnosis
Apr 09, 2024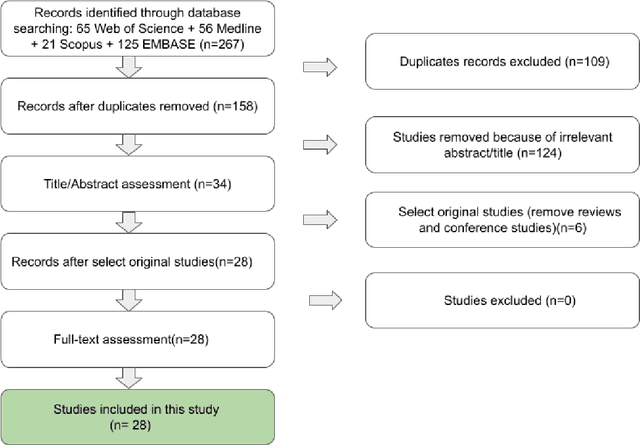
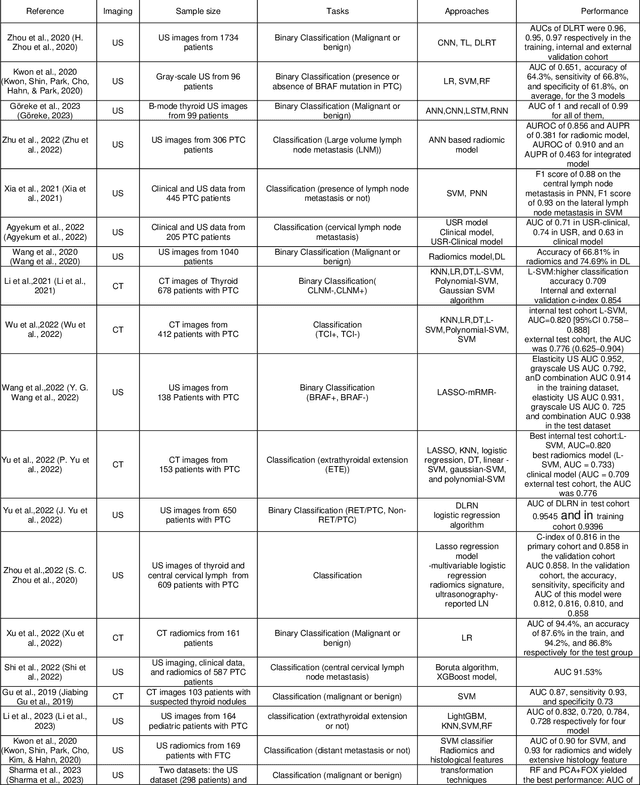
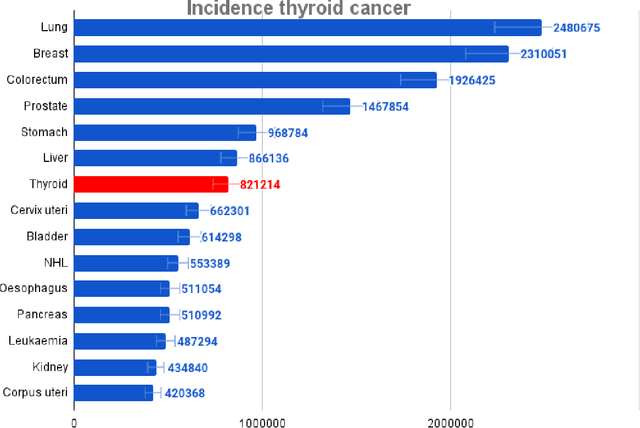
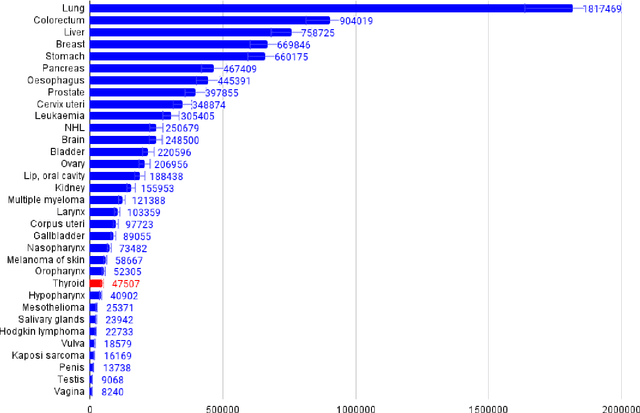
Abstract:Thyroid cancer is an increasing global health concern that requires advanced diagnostic methods. The application of AI and radiomics to thyroid cancer diagnosis is examined in this review. A review of multiple databases was conducted in compliance with PRISMA guidelines until October 2023. A combination of keywords led to the discovery of an English academic publication on thyroid cancer and related subjects. 267 papers were returned from the original search after 109 duplicates were removed. Relevant studies were selected according to predetermined criteria after 124 articles were eliminated based on an examination of their abstract and title. After the comprehensive analysis, an additional six studies were excluded. Among the 28 included studies, radiomics analysis, which incorporates ultrasound (US) images, demonstrated its effectiveness in diagnosing thyroid cancer. Various results were noted, some of the studies presenting new strategies that outperformed the status quo. The literature has emphasized various challenges faced by AI models, including interpretability issues, dataset constraints, and operator dependence. The synthesized findings of the 28 included studies mentioned the need for standardization efforts and prospective multicenter studies to address these concerns. Furthermore, approaches to overcome these obstacles were identified, such as advances in explainable AI technology and personalized medicine techniques. The review focuses on how AI and radiomics could transform the diagnosis and treatment of thyroid cancer. Despite challenges, future research on multidisciplinary cooperation, clinical applicability validation, and algorithm improvement holds the potential to improve patient outcomes and diagnostic precision in the treatment of thyroid cancer.
Artificial Intelligence and Diabetes Mellitus: An Inside Look Through the Retina
Feb 28, 2024



Abstract:Diabetes mellitus (DM) predisposes patients to vascular complications. Retinal images and vasculature reflect the body's micro- and macrovascular health. They can be used to diagnose DM complications, including diabetic retinopathy (DR), neuropathy, nephropathy, and atherosclerotic cardiovascular disease, as well as forecast the risk of cardiovascular events. Artificial intelligence (AI)-enabled systems developed for high-throughput detection of DR using digitized retinal images have become clinically adopted. Beyond DR screening, AI integration also holds immense potential to address challenges associated with the holistic care of the patient with DM. In this work, we aim to comprehensively review the literature for studies on AI applications based on retinal images related to DM diagnosis, prognostication, and management. We will describe the findings of holistic AI-assisted diabetes care, including but not limited to DR screening, and discuss barriers to implementing such systems, including issues concerning ethics, data privacy, equitable access, and explainability. With the ability to evaluate the patient's health status vis a vis DM complication as well as risk prognostication of future cardiovascular complications, AI-assisted retinal image analysis has the potential to become a central tool for modern personalized medicine in patients with DM.
Current and future roles of artificial intelligence in retinopathy of prematurity
Feb 15, 2024


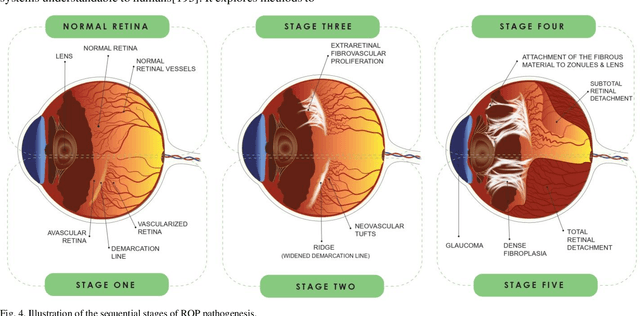
Abstract:Retinopathy of prematurity (ROP) is a severe condition affecting premature infants, leading to abnormal retinal blood vessel growth, retinal detachment, and potential blindness. While semi-automated systems have been used in the past to diagnose ROP-related plus disease by quantifying retinal vessel features, traditional machine learning (ML) models face challenges like accuracy and overfitting. Recent advancements in deep learning (DL), especially convolutional neural networks (CNNs), have significantly improved ROP detection and classification. The i-ROP deep learning (i-ROP-DL) system also shows promise in detecting plus disease, offering reliable ROP diagnosis potential. This research comprehensively examines the contemporary progress and challenges associated with using retinal imaging and artificial intelligence (AI) to detect ROP, offering valuable insights that can guide further investigation in this domain. Based on 89 original studies in this field (out of 1487 studies that were comprehensively reviewed), we concluded that traditional methods for ROP diagnosis suffer from subjectivity and manual analysis, leading to inconsistent clinical decisions. AI holds great promise for improving ROP management. This review explores AI's potential in ROP detection, classification, diagnosis, and prognosis.
Artificial Intelligence in Assessing Cardiovascular Diseases and Risk Factors via Retinal Fundus Images: A Review of the Last Decade
Nov 11, 2023



Abstract:Background: Cardiovascular diseases (CVDs) continue to be the leading cause of mortality on a global scale. In recent years, the application of artificial intelligence (AI) techniques, particularly deep learning (DL), has gained considerable popularity for evaluating the various aspects of CVDs. Moreover, using fundus images and optical coherence tomography angiography (OCTA) to diagnose retinal diseases has been extensively studied. To better understand heart function and anticipate changes based on microvascular characteristics and function, researchers are currently exploring the integration of AI with non-invasive retinal scanning. Leveraging AI-assisted early detection and prediction of cardiovascular diseases on a large scale holds excellent potential to mitigate cardiovascular events and alleviate the economic burden on healthcare systems. Method: A comprehensive search was conducted across various databases, including PubMed, Medline, Google Scholar, Scopus, Web of Sciences, IEEE Xplore, and ACM Digital Library, using specific keywords related to cardiovascular diseases and artificial intelligence. Results: A total of 87 English-language publications, selected for relevance were included in the study, and additional references were considered. This study presents an overview of the current advancements and challenges in employing retinal imaging and artificial intelligence to identify cardiovascular disorders and provides insights for further exploration in this field. Conclusion: Researchers aim to develop precise disease prognosis patterns as the aging population and global CVD burden increase. AI and deep learning are transforming healthcare, offering the potential for single retinal image-based diagnosis of various CVDs, albeit with the need for accelerated adoption in healthcare systems.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge