Pawel Plawiak
Advancements in Radiomics and Artificial Intelligence for Thyroid Cancer Diagnosis
Apr 09, 2024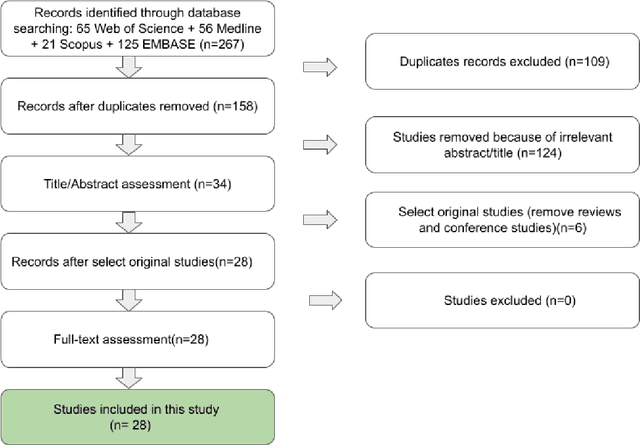
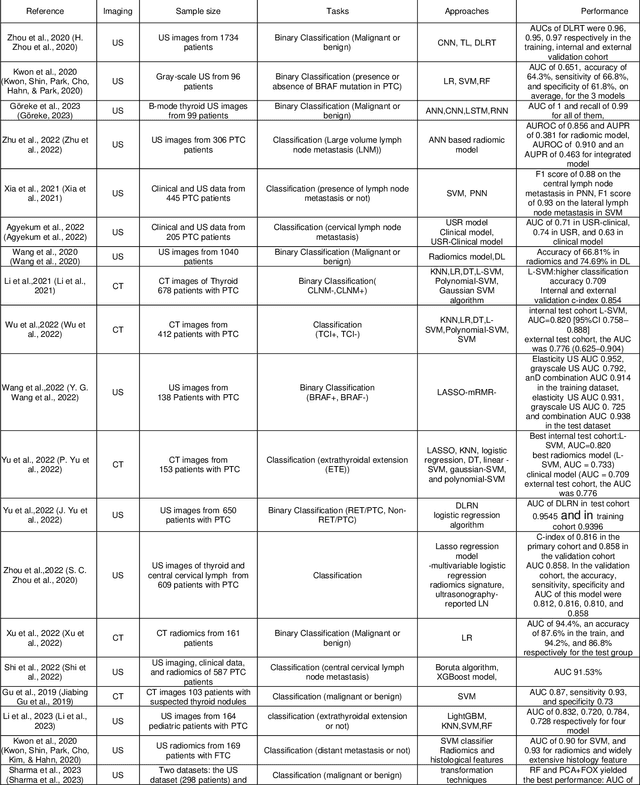
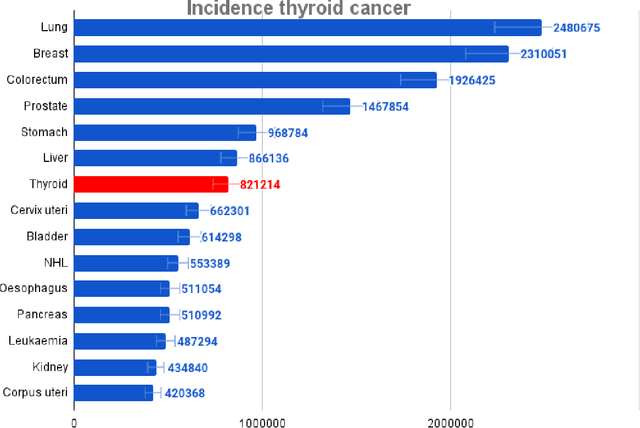
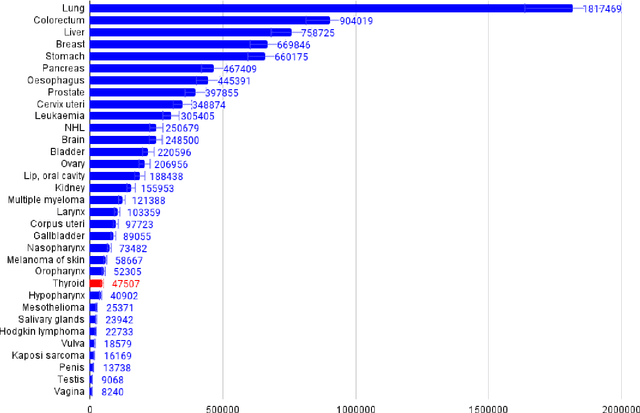
Abstract:Thyroid cancer is an increasing global health concern that requires advanced diagnostic methods. The application of AI and radiomics to thyroid cancer diagnosis is examined in this review. A review of multiple databases was conducted in compliance with PRISMA guidelines until October 2023. A combination of keywords led to the discovery of an English academic publication on thyroid cancer and related subjects. 267 papers were returned from the original search after 109 duplicates were removed. Relevant studies were selected according to predetermined criteria after 124 articles were eliminated based on an examination of their abstract and title. After the comprehensive analysis, an additional six studies were excluded. Among the 28 included studies, radiomics analysis, which incorporates ultrasound (US) images, demonstrated its effectiveness in diagnosing thyroid cancer. Various results were noted, some of the studies presenting new strategies that outperformed the status quo. The literature has emphasized various challenges faced by AI models, including interpretability issues, dataset constraints, and operator dependence. The synthesized findings of the 28 included studies mentioned the need for standardization efforts and prospective multicenter studies to address these concerns. Furthermore, approaches to overcome these obstacles were identified, such as advances in explainable AI technology and personalized medicine techniques. The review focuses on how AI and radiomics could transform the diagnosis and treatment of thyroid cancer. Despite challenges, future research on multidisciplinary cooperation, clinical applicability validation, and algorithm improvement holds the potential to improve patient outcomes and diagnostic precision in the treatment of thyroid cancer.
RECOMMED: A Comprehensive Pharmaceutical Recommendation System
Dec 31, 2022



Abstract:A comprehensive pharmaceutical recommendation system was designed based on the patients and drugs features extracted from Drugs.com and Druglib.com. First, data from these databases were combined, and a dataset of patients and drug information was built. Secondly, the patients and drugs were clustered, and then the recommendation was performed using different ratings provided by patients, and importantly by the knowledge obtained from patients and drug specifications, and considering drug interactions. To the best of our knowledge, we are the first group to consider patients conditions and history in the proposed approach for selecting a specific medicine appropriate for that particular user. Our approach applies artificial intelligence (AI) models for the implementation. Sentiment analysis using natural language processing approaches is employed in pre-processing along with neural network-based methods and recommender system algorithms for modeling the system. In our work, patients conditions and drugs features are used for making two models based on matrix factorization. Then we used drug interaction to filter drugs with severe or mild interactions with other drugs. We developed a deep learning model for recommending drugs by using data from 2304 patients as a training set, and then we used data from 660 patients as our validation set. After that, we used knowledge from critical information about drugs and combined the outcome of the model into a knowledge-based system with the rules obtained from constraints on taking medicine.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge