Shelly Soffer
Weakly Supervised Attention Model for RV StrainClassification from volumetric CTPA Scans
Jul 26, 2021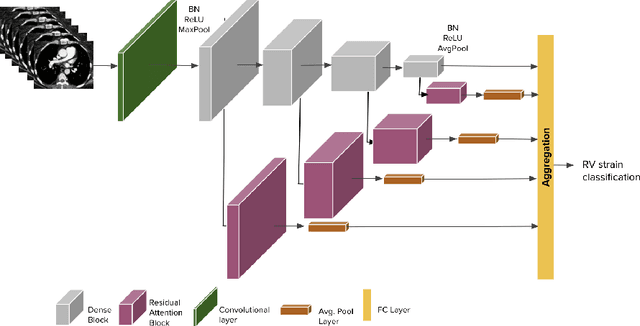
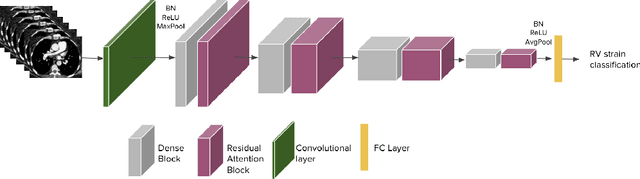
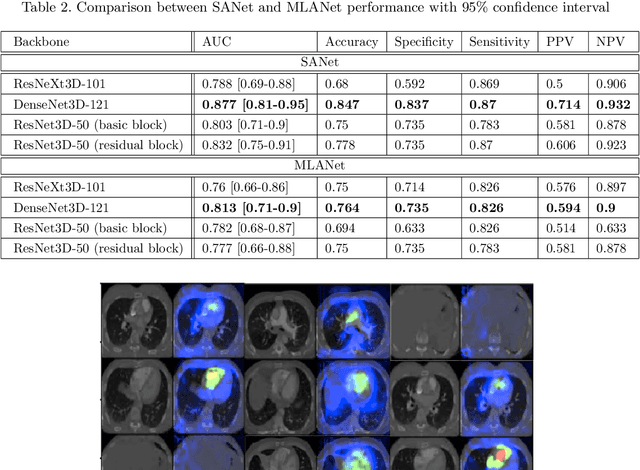
Abstract:Pulmonary embolus (PE) refers to obstruction of pulmonary arteries by blood clots. PE accounts for approximately 100,000 deaths per year in the United States alone. The clinical presentation of PE is often nonspecific, making the diagnosis challenging. Thus, rapid and accurate risk stratification is of paramount importance. High-risk PE is caused by right ventricular (RV) dysfunction from acute pressure overload, which in return can help identify which patients require more aggressive therapy. Reconstructed four-chamber views of the heart on chest CT can detect right ventricular enlargement. CT pulmonary angiography (CTPA) is the golden standard in the diagnostic workup of suspected PE. Therefore, it can link between diagnosis and risk stratification strategies. We developed a weakly supervised deep learning algorithm, with an emphasis on a novel attention mechanism, to automatically classify RV strain on CTPA. Our method is a 3D DenseNet model with integrated 3D residual attention blocks. We evaluated our model on a dataset of CTPAs of emergency department (ED) PE patients. This model achieved an area under the receiver operating characteristic curve (AUC) of 0.88 for classifying RV strain. The model showed a sensitivity of 87% and specificity of 83.7%. Our solution outperforms state-of-the-art 3D CNN networks. The proposed design allows for a fully automated network that can be trained easily in an end-to-end manner without requiring computationally intensive and time-consuming preprocessing or strenuous labeling of the data.We infer that unmarked CTPAs can be used for effective RV strain classification. This could be used as a second reader, alerting for high-risk PE patients. To the best of our knowledge, there are no previous deep learning-based studies that attempted to solve this problem.
Cross-Modality Synthesis from CT to PET using FCN and GAN Networks for Improved Automated Lesion Detection
Jul 23, 2018



Abstract:In this work we present a novel system for generation of virtual PET images using CT scans. We combine a fully convolutional network (FCN) with a conditional generative adversarial network (GAN) to generate simulated PET data from given input CT data. The synthesized PET can be used for false-positive reduction in lesion detection solutions. Clinically, such solutions may enable lesion detection and drug treatment evaluation in a CT-only environment, thus reducing the need for the more expensive and radioactive PET/CT scan. Our dataset includes 60 PET/CT scans from Sheba Medical center. We used 23 scans for training and 37 for testing. Different schemes to achieve the synthesized output were qualitatively compared. Quantitative evaluation was conducted using an existing lesion detection software, combining the synthesized PET as a false positive reduction layer for the detection of malignant lesions in the liver. Current results look promising showing a 28% reduction in the average false positive per case from 2.9 to 2.1. The suggested solution is comprehensive and can be expanded to additional body organs, and different modalities.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge