Sepideh Maleki
HyperQuery: Beyond Binary Link Prediction
Jan 13, 2025



Abstract:Groups with complex set intersection relations are a natural way to model a wide array of data, from the formation of social groups to the complex protein interactions which form the basis of biological life. One approach to representing such higher order relationships is as a hypergraph. However, efforts to apply machine learning techniques to hypergraph structured datasets have been limited thus far. In this paper, we address the problem of link prediction in knowledge hypergraphs as well as simple hypergraphs and develop a novel, simple, and effective optimization architecture that addresses both tasks. Additionally, we introduce a novel feature extraction technique using node level clustering and we show how integrating data from node-level labels can improve system performance. Our self-supervised approach achieves significant improvement over state of the art baselines on several hyperedge prediction and knowledge hypergraph completion benchmarks.
Efficient Fine-Tuning of Single-Cell Foundation Models Enables Zero-Shot Molecular Perturbation Prediction
Dec 18, 2024



Abstract:Predicting transcriptional responses to novel drugs provides a unique opportunity to accelerate biomedical research and advance drug discovery efforts. However, the inherent complexity and high dimensionality of cellular responses, combined with the extremely limited available experimental data, makes the task challenging. In this study, we leverage single-cell foundation models (FMs) pre-trained on tens of millions of single cells, encompassing multiple cell types, states, and disease annotations, to address molecular perturbation prediction. We introduce a drug-conditional adapter that allows efficient fine-tuning by training less than 1% of the original foundation model, thus enabling molecular conditioning while preserving the rich biological representation learned during pre-training. The proposed strategy allows not only the prediction of cellular responses to novel drugs, but also the zero-shot generalization to unseen cell lines. We establish a robust evaluation framework to assess model performance across different generalization tasks, demonstrating state-of-the-art results across all settings, with significant improvements in the few-shot and zero-shot generalization to new cell lines compared to existing baselines.
NetVec: A Scalable Hypergraph Embedding System
Mar 09, 2021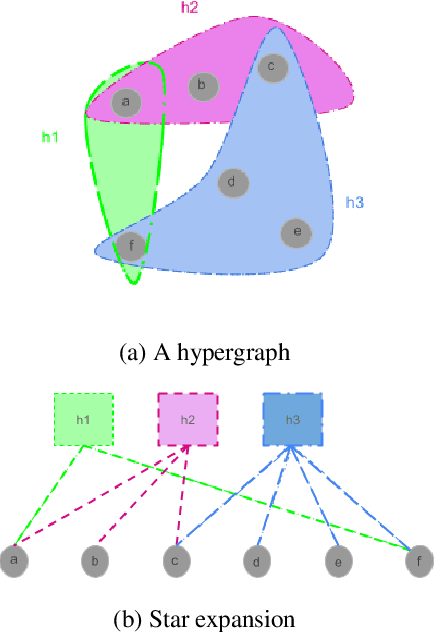
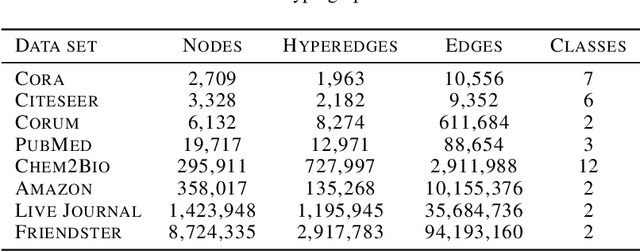
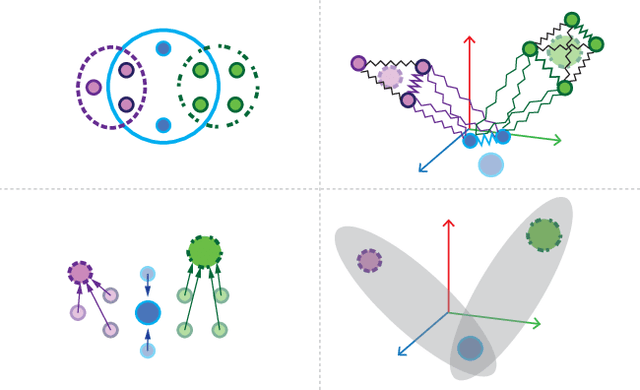

Abstract:Many problems such as vertex classification andlink prediction in network data can be solvedusing graph embeddings, and a number of algo-rithms are known for constructing such embed-dings. However, it is difficult to use graphs tocapture non-binary relations such as communitiesof vertices. These kinds of complex relations areexpressed more naturally as hypergraphs. Whilehypergraphs are a generalization of graphs, state-of-the-art graph embedding techniques are notadequate for solving prediction and classificationtasks on large hypergraphs accurately in reason-able time. In this paper, we introduce NetVec,a novel multi-level framework for scalable un-supervised hypergraph embedding, that can becoupled with any graph embedding algorithm toproduce embeddings of hypergraphs with millionsof nodes and hyperedges in a few minutes.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge