Robin Lemmens
Dice Semimetric Losses: Optimizing the Dice Score with Soft Labels
Apr 01, 2023



Abstract:The soft Dice loss (SDL) has taken a pivotal role in many automated segmentation pipelines in the medical imaging community. Over the last years, some reasons behind its superior functioning have been uncovered and further optimizations have been explored. However, there is currently no implementation that supports its direct use in settings with soft labels. Hence, a synergy between the use of SDL and research leveraging the use of soft labels, also in the context of model calibration, is still missing. In this work, we introduce Dice semimetric losses (DMLs), which (i) are by design identical to SDL in a standard setting with hard labels, but (ii) can be used in settings with soft labels. Our experiments on the public QUBIQ, LiTS and KiTS benchmarks confirm the potential synergy of DMLs with soft labels (e.g. averaging, label smoothing, and knowledge distillation) over hard labels (e.g. majority voting and random selection). As a result, we obtain superior Dice scores and model calibration, which supports the wider adoption of DMLs in practice. Code is available at \href{https://github.com/zifuwanggg/JDTLosses}{https://github.com/zifuwanggg/JDTLosses}.
Convolutional neural networks for medical image segmentation
Nov 17, 2022



Abstract:In this article, we look into some essential aspects of convolutional neural networks (CNNs) with the focus on medical image segmentation. First, we discuss the CNN architecture, thereby highlighting the spatial origin of the data, voxel-wise classification and the receptive field. Second, we discuss the sampling of input-output pairs, thereby highlighting the interaction between voxel-wise classification, patch size and the receptive field. Finally, we give a historical overview of crucial changes to CNN architectures for classification and segmentation, giving insights in the relation between three pivotal CNN architectures: FCN, U-Net and DeepMedic.
DeepVoxNet2: Yet another CNN framework
Nov 17, 2022Abstract:We know that both the CNN mapping function and the sampling scheme are of paramount importance for CNN-based image analysis. It is clear that both functions operate in the same space, with an image axis $\mathcal{I}$ and a feature axis $\mathcal{F}$. Remarkably, we found that no frameworks existed that unified the two and kept track of the spatial origin of the data automatically. Based on our own practical experience, we found the latter to often result in complex coding and pipelines that are difficult to exchange. This article introduces our framework for 1, 2 or 3D image classification or segmentation: DeepVoxNet2 (DVN2). This article serves as an interactive tutorial, and a pre-compiled version, including the outputs of the code blocks, can be found online in the public DVN2 repository. This tutorial uses data from the multimodal Brain Tumor Image Segmentation Benchmark (BRATS) of 2018 to show an example of a 3D segmentation pipeline.
Final infarct prediction in acute ischemic stroke
Nov 09, 2022Abstract:This article focuses on the control center of each human body: the brain. We will point out the pivotal role of the cerebral vasculature and how its complex mechanisms may vary between subjects. We then emphasize a specific acute pathological state, i.e., acute ischemic stroke, and show how medical imaging and its analysis can be used to define the treatment. We show how the core-penumbra concept is used in practice using mismatch criteria and how machine learning can be used to make predictions of the final infarct, either via deconvolution or convolutional neural networks.
Prediction of final infarct volume from native CT perfusion and treatment parameters using deep learning
Dec 06, 2018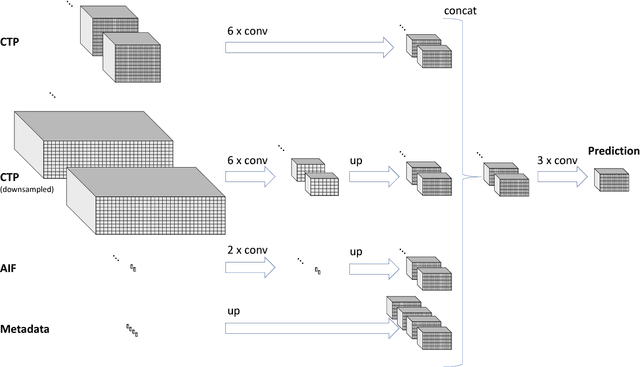
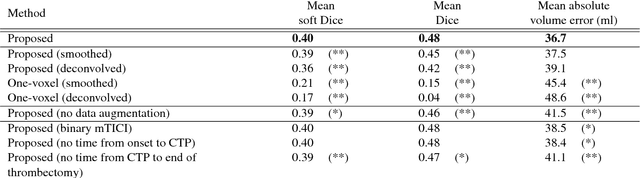
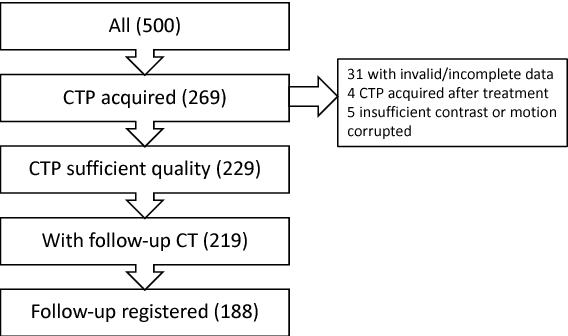
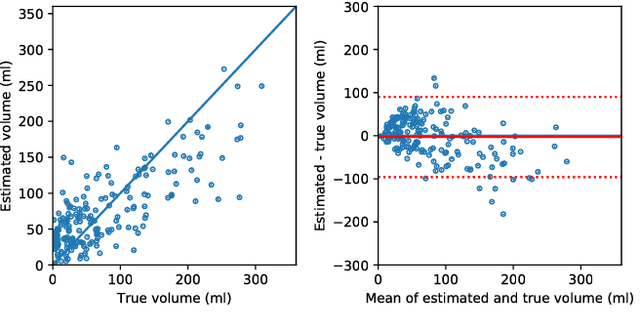
Abstract:CT Perfusion (CTP) imaging has gained importance in the diagnosis of acute stroke. Conventional perfusion analysis performs a deconvolution of the measurements and thresholds the perfusion parameters to determine the tissue status. We pursue a data-driven and deconvolution-free approach, where a deep neural network learns to predict the final infarct volume directly from the native CTP images and metadata such as the time parameters and treatment. This would allow clinicians to simulate various treatments and gain insight into predicted tissue status over time. We demonstrate on a multicenter dataset that our approach is able to predict the final infarct and effectively uses the metadata. An ablation study shows that using the native CTP measurements instead of the deconvolved measurements improves the prediction.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge