Robin A. de Graaf
A Flow-based Truncated Denoising Diffusion Model for Super-resolution Magnetic Resonance Spectroscopic Imaging
Oct 25, 2024Abstract:Magnetic Resonance Spectroscopic Imaging (MRSI) is a non-invasive imaging technique for studying metabolism and has become a crucial tool for understanding neurological diseases, cancers and diabetes. High spatial resolution MRSI is needed to characterize lesions, but in practice MRSI is acquired at low resolution due to time and sensitivity restrictions caused by the low metabolite concentrations. Therefore, there is an imperative need for a post-processing approach to generate high-resolution MRSI from low-resolution data that can be acquired fast and with high sensitivity. Deep learning-based super-resolution methods provided promising results for improving the spatial resolution of MRSI, but they still have limited capability to generate accurate and high-quality images. Recently, diffusion models have demonstrated superior learning capability than other generative models in various tasks, but sampling from diffusion models requires iterating through a large number of diffusion steps, which is time-consuming. This work introduces a Flow-based Truncated Denoising Diffusion Model (FTDDM) for super-resolution MRSI, which shortens the diffusion process by truncating the diffusion chain, and the truncated steps are estimated using a normalizing flow-based network. The network is conditioned on upscaling factors to enable multi-scale super-resolution. To train and evaluate the deep learning models, we developed a 1H-MRSI dataset acquired from 25 high-grade glioma patients. We demonstrate that FTDDM outperforms existing generative models while speeding up the sampling process by over 9-fold compared to the baseline diffusion model. Neuroradiologists' evaluations confirmed the clinical advantages of our method, which also supports uncertainty estimation and sharpness adjustment, extending its potential clinical applications.
* Accepted by Medical Image Analysis (MedIA)
Preserved Edge Convolutional Neural Network for Sensitivity Enhancement of Deuterium Metabolic Imaging (DMI)
Sep 13, 2023
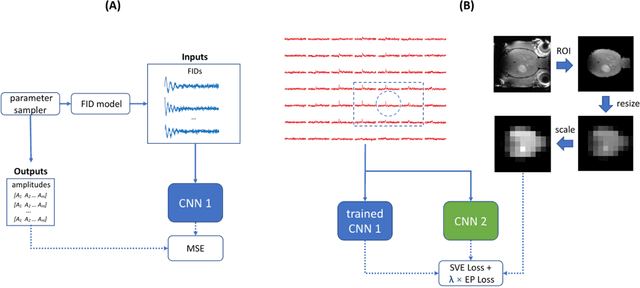
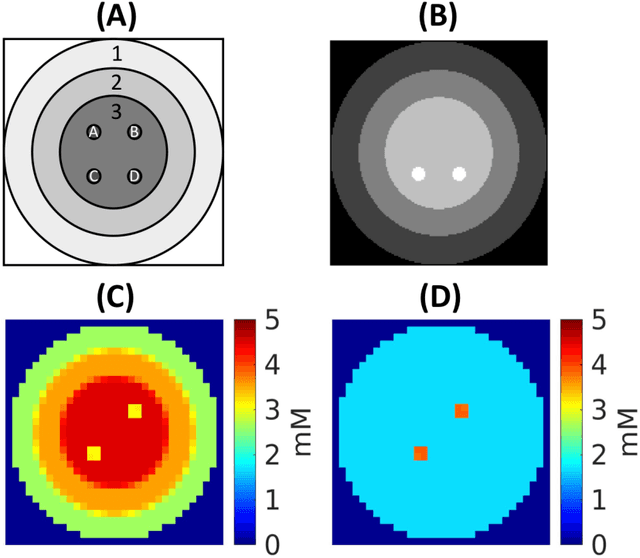
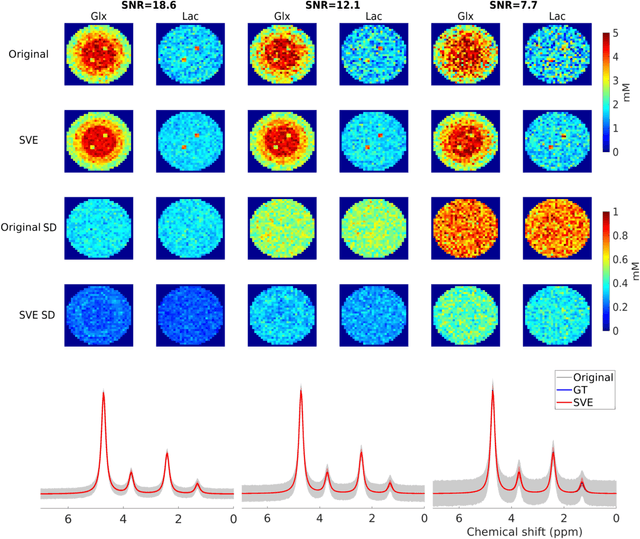
Abstract:Purpose: Common to most MRSI techniques, the spatial resolution and the minimal scan duration of Deuterium Metabolic Imaging (DMI) are limited by the achievable SNR. This work presents a deep learning method for sensitivity enhancement of DMI. Methods: A convolutional neural network (CNN) was designed to estimate the 2H-labeled metabolite concentrations from low SNR and distorted DMI FIDs. The CNN was trained with synthetic data that represent a range of SNR levels typically encountered in vivo. The estimation precision was further improved by fine-tuning the CNN with MRI-based edge-preserving regularization for each DMI dataset. The proposed processing method, PReserved Edge ConvolutIonal neural network for Sensitivity Enhanced DMI (PRECISE-DMI), was applied to simulation studies and in vivo experiments to evaluate the anticipated improvements in SNR and investigate the potential for inaccuracies. Results: PRECISE-DMI visually improved the metabolic maps of low SNR datasets, and quantitatively provided higher precision than the standard Fourier reconstruction. Processing of DMI data acquired in rat brain tumor models resulted in more precise determination of 2H-labeled lactate and glutamate + glutamine levels, at increased spatial resolution (from >8 to 2 $\mu$L) or shortened scan time (from 32 to 4 min) compared to standard acquisitions. However, rigorous SD-bias analyses showed that overuse of the edge-preserving regularization can compromise the accuracy of the results. Conclusion: PRECISE-DMI allows a flexible trade-off between enhancing the sensitivity of DMI and minimizing the inaccuracies. With typical settings, the DMI sensitivity can be improved by 3-fold while retaining the capability to detect local signal variations.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge