Paul Koomey
The State of Applying Artificial Intelligence to Tissue Imaging for Cancer Research and Early Detection
Jun 29, 2023



Abstract:Artificial intelligence represents a new frontier in human medicine that could save more lives and reduce the costs, thereby increasing accessibility. As a consequence, the rate of advancement of AI in cancer medical imaging and more particularly tissue pathology has exploded, opening it to ethical and technical questions that could impede its adoption into existing systems. In order to chart the path of AI in its application to cancer tissue imaging, we review current work and identify how it can improve cancer pathology diagnostics and research. In this review, we identify 5 core tasks that models are developed for, including regression, classification, segmentation, generation, and compression tasks. We address the benefits and challenges that such methods face, and how they can be adapted for use in cancer prevention and treatment. The studies looked at in this paper represent the beginning of this field and future experiments will build on the foundations that we highlight.
Clinically Relevant Latent Space Embedding of Cancer Histopathology Slides through Variational Autoencoder Based Image Compression
Mar 23, 2023Abstract:In this paper, we introduce a Variational Autoencoder (VAE) based training approach that can compress and decompress cancer pathology slides at a compression ratio of 1:512, which is better than the previously reported state of the art (SOTA) in the literature, while still maintaining accuracy in clinical validation tasks. The compression approach was tested on more common computer vision datasets such as CIFAR10, and we explore which image characteristics enable this compression ratio on cancer imaging data but not generic images. We generate and visualize embeddings from the compressed latent space and demonstrate how they are useful for clinical interpretation of data, and how in the future such latent embeddings can be used to accelerate search of clinical imaging data.
A SSIM Guided cGAN Architecture For Clinically Driven Generative Image Synthesis of Multiplexed Spatial Proteomics Channels
May 20, 2022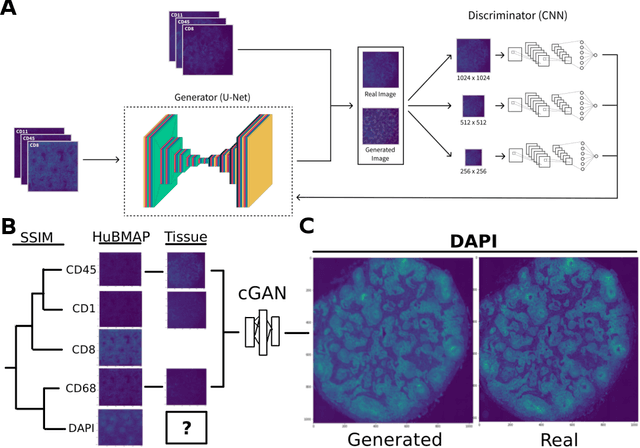
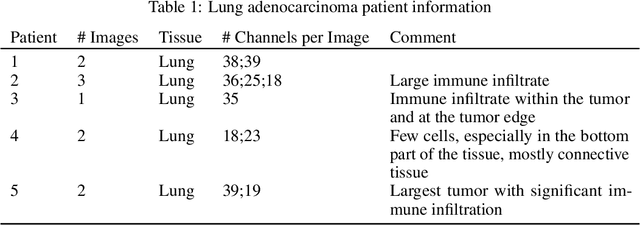

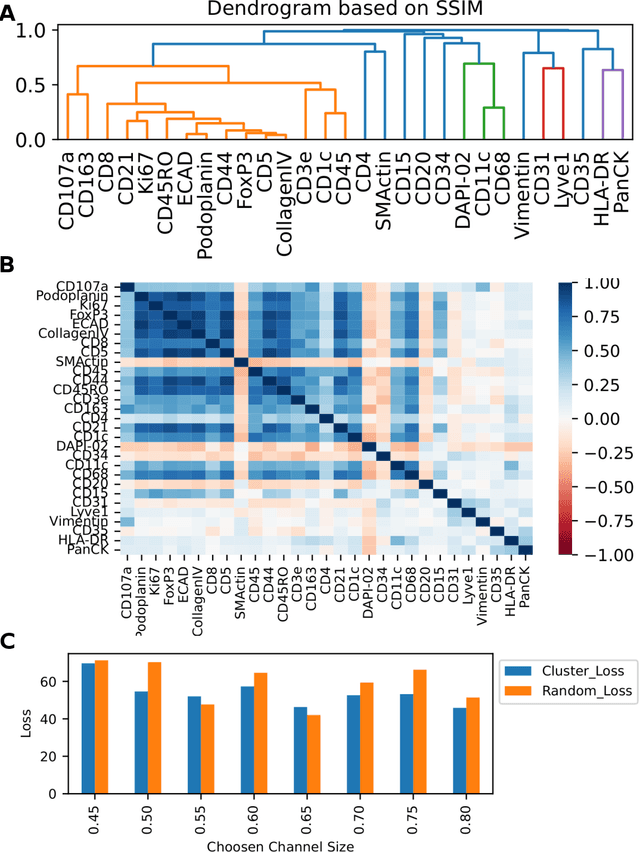
Abstract:Here we present a structural similarity index measure (SSIM) guided conditional Generative Adversarial Network (cGAN) that generatively performs image-to-image (i2i) synthesis to generate photo-accurate protein channels in multiplexed spatial proteomics images. This approach can be utilized to accurately generate missing spatial proteomics channels that were not included during experimental data collection either at the bench or the clinic. Experimental spatial proteomic data from the Human BioMolecular Atlas Program (HuBMAP) was used to generate spatial representations of missing proteins through a U-Net based image synthesis pipeline. HuBMAP channels were hierarchically clustered by the (SSIM) as a heuristic to obtain the minimal set needed to recapitulate the underlying biology represented by the spatial landscape of proteins. We subsequently prove that our SSIM based architecture allows for scaling of generative image synthesis to slides with up to 100 channels, which is better than current state of the art algorithms which are limited to data with 11 channels. We validate these claims by generating a new experimental spatial proteomics data set from human lung adenocarcinoma tissue sections and show that a model trained on HuBMAP can accurately synthesize channels from our new data set. The ability to recapitulate experimental data from sparsely stained multiplexed histological slides containing spatial proteomic will have tremendous impact on medical diagnostics and drug development, and also raises important questions on the medical ethics of utilizing data produced by generative image synthesis in the clinical setting. The algorithm that we present in this paper will allow researchers and clinicians to save time and costs in proteomics based histological staining while also increasing the amount of data that they can generate through their experiments.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge