Or Bar-Shira
Deep Algorithm Unrolling for Biomedical Imaging
Aug 15, 2021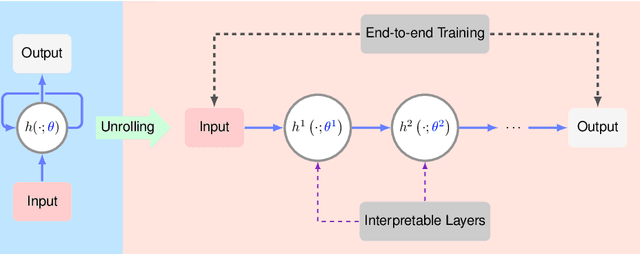
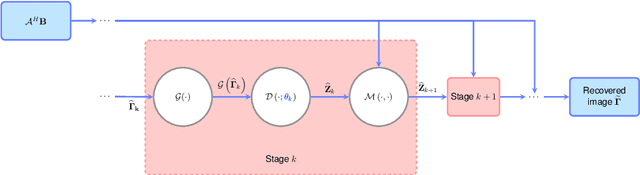
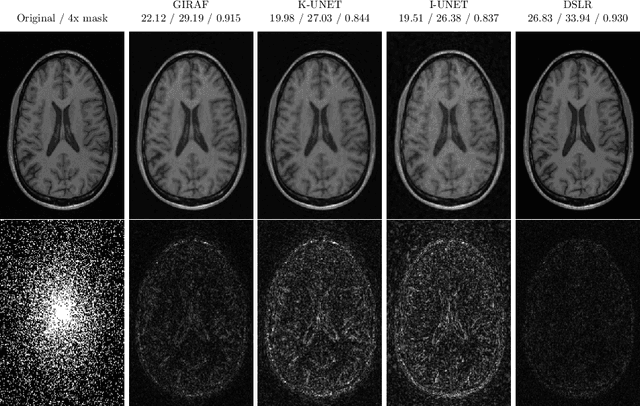
Abstract:In this chapter, we review biomedical applications and breakthroughs via leveraging algorithm unrolling, an important technique that bridges between traditional iterative algorithms and modern deep learning techniques. To provide context, we start by tracing the origin of algorithm unrolling and providing a comprehensive tutorial on how to unroll iterative algorithms into deep networks. We then extensively cover algorithm unrolling in a wide variety of biomedical imaging modalities and delve into several representative recent works in detail. Indeed, there is a rich history of iterative algorithms for biomedical image synthesis, which makes the field ripe for unrolling techniques. In addition, we put algorithm unrolling into a broad perspective, in order to understand why it is particularly effective and discuss recent trends. Finally, we conclude the chapter by discussing open challenges, and suggesting future research directions.
Learned super resolution ultrasound for improved breast lesion characterization
Jul 12, 2021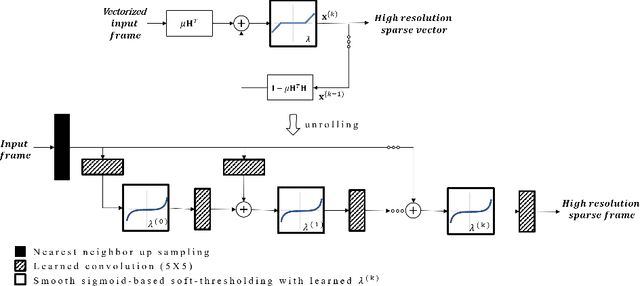
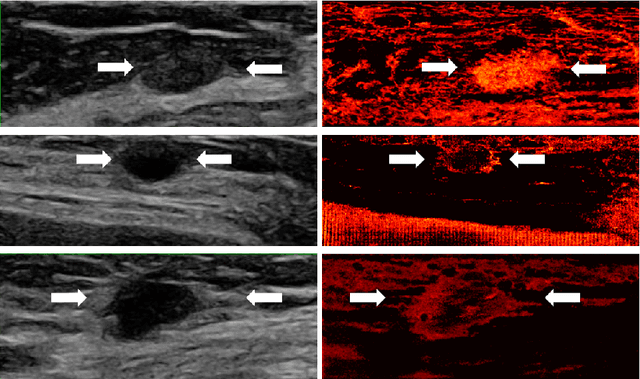
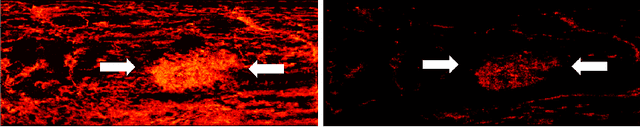
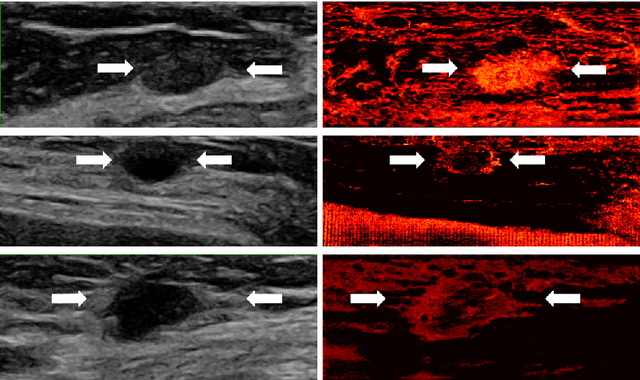
Abstract:Breast cancer is the most common malignancy in women. Mammographic findings such as microcalcifications and masses, as well as morphologic features of masses in sonographic scans, are the main diagnostic targets for tumor detection. However, improved specificity of these imaging modalities is required. A leading alternative target is neoangiogenesis. When pathological, it contributes to the development of numerous types of tumors, and the formation of metastases. Hence, demonstrating neoangiogenesis by visualization of the microvasculature may be of great importance. Super resolution ultrasound localization microscopy enables imaging of the microvasculature at the capillary level. Yet, challenges such as long reconstruction time, dependency on prior knowledge of the system Point Spread Function (PSF), and separability of the Ultrasound Contrast Agents (UCAs), need to be addressed for translation of super-resolution US into the clinic. In this work we use a deep neural network architecture that makes effective use of signal structure to address these challenges. We present in vivo human results of three different breast lesions acquired with a clinical US scanner. By leveraging our trained network, the microvasculature structure is recovered in a short time, without prior PSF knowledge, and without requiring separability of the UCAs. Each of the recoveries exhibits a different structure that corresponds with the known histological structure. This study demonstrates the feasibility of in vivo human super resolution, based on a clinical scanner, to increase US specificity for different breast lesions and promotes the use of US in the diagnosis of breast pathologies.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge