Nikolaos V. Tsekos
Two Deep Learning Approaches for Automated Segmentation of Left Ventricle in Cine Cardiac MRI
Jan 02, 2026Abstract:Left ventricle (LV) segmentation is critical for clinical quantification and diagnosis of cardiac images. In this work, we propose two novel deep learning architectures called LNU-Net and IBU-Net for left ventricle segmentation from short-axis cine MRI images. LNU-Net is derived from layer normalization (LN) U-Net architecture, while IBU-Net is derived from the instance-batch normalized (IB) U-Net for medical image segmentation. The architectures of LNU-Net and IBU-Net have a down-sampling path for feature extraction and an up-sampling path for precise localization. We use the original U-Net as the basic segmentation approach and compared it with our proposed architectures. Both LNU-Net and IBU-Net have left ventricle segmentation methods: LNU-Net applies layer normalization in each convolutional block, while IBU-Net incorporates instance and batch normalization together in the first convolutional block and passes its result to the next layer. Our method incorporates affine transformations and elastic deformations for image data processing. Our dataset that contains 805 MRI images regarding the left ventricle from 45 patients is used for evaluation. We experimentally evaluate the results of the proposed approaches outperforming the dice coefficient and the average perpendicular distance than other state-of-the-art approaches.
* 7 pages, 5 figures, published in ICBBB 2022
Simulations of MRI Guided and Powered Ferric Applicators for Tetherless Delivery of Therapeutic Interventions
Jan 02, 2026Abstract:Magnetic Resonance Imaging (MRI) is a well-established modality for pre-operative planning and is also explored for intra-operative guidance of procedures such as intravascular interventions. Among the experimental robot-assisted technologies, the magnetic field gradients of the MRI scanner are used to power and maneuver ferromagnetic applicators for accessing sites in the patient's body via the vascular network. In this work, we propose a computational platform for preoperative planning and modeling of MRI-powered applicators inside blood vessels. This platform was implemented as a two-way data and command pipeline that links the MRI scanner, the computational core, and the operator. The platform first processes multi-slice MR data to extract the vascular bed and then fits a virtual corridor inside the vessel. This corridor serves as a virtual fixture (VF), a forbidden region for the applicators to avoid vessel perforation or collision. The geometric features of the vessel centerline, the VF, and MRI safety compliance (dB/dt, max available gradient) are then used to generate magnetic field gradient waveforms. Different blood flow profiles can be user-selected, and those parameters are used for modeling the applicator's maneuvering. The modeling module further generates cues about whether the selected vascular path can be safely maneuvered. Given future experimental studies that require a real-time operation, the platform was implemented on the Qt framework (C/C++) with software modules performing specific tasks running on dedicated threads: PID controller, generation of VF, generation of MR gradient waveforms.
* 9 pages, 8 figures, published in ICBBB 2022
Deep Learning methods for automatic evaluation of delayed enhancement-MRI. The results of the EMIDEC challenge
Aug 10, 2021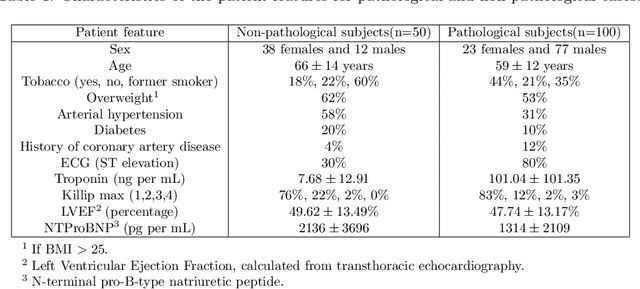
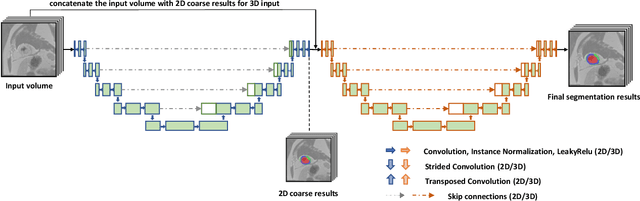
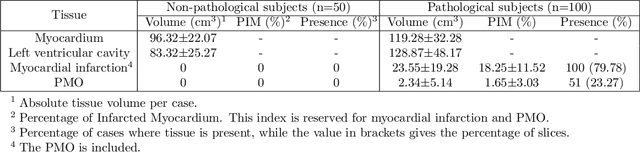
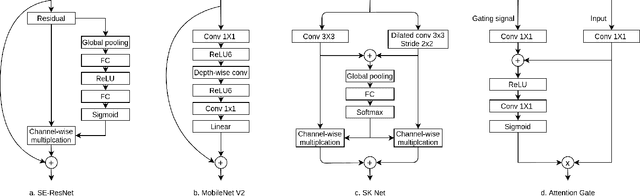
Abstract:A key factor for assessing the state of the heart after myocardial infarction (MI) is to measure whether the myocardium segment is viable after reperfusion or revascularization therapy. Delayed enhancement-MRI or DE-MRI, which is performed several minutes after injection of the contrast agent, provides high contrast between viable and nonviable myocardium and is therefore a method of choice to evaluate the extent of MI. To automatically assess myocardial status, the results of the EMIDEC challenge that focused on this task are presented in this paper. The challenge's main objectives were twofold. First, to evaluate if deep learning methods can distinguish between normal and pathological cases. Second, to automatically calculate the extent of myocardial infarction. The publicly available database consists of 150 exams divided into 50 cases with normal MRI after injection of a contrast agent and 100 cases with myocardial infarction (and then with a hyperenhanced area on DE-MRI), whatever their inclusion in the cardiac emergency department. Along with MRI, clinical characteristics are also provided. The obtained results issued from several works show that the automatic classification of an exam is a reachable task (the best method providing an accuracy of 0.92), and the automatic segmentation of the myocardium is possible. However, the segmentation of the diseased area needs to be improved, mainly due to the small size of these areas and the lack of contrast with the surrounding structures.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge