Nhan Duy Truong
Prospective Identification of Ictal Electroencephalogram
Mar 03, 2021


Abstract:A vast majority of epileptic seizure (ictal) detection on electroencephalogram (EEG) data has been retrospective. Therefore, even though some may include many patients and extensive evaluation benchmarking, they all share a heavy reliance on labelled data. This is perhaps the most significant obstacle against the utility of seizure detection systems in clinical settings. In this paper, we present a prospective automatic ictal detection and labelling performed at the level of a human expert (arbiter) and reduces labelling time by more than an order of magnitude. Accurate seizure detection and labelling are still a time-consuming and cumbersome task in epilepsy monitoring units (EMUs) and epilepsy centres, particularly in countries with limited facilities and insufficiently trained human resources. This work implements a convolutional long short-term memory (ConvLSTM) network that is pre-trained and tested on Temple University Hospital (TUH) EEG corpus. It is then deployed prospectively at the Comprehensive Epilepsy Service at the Royal Prince Alfred Hospital (RPAH) in Sydney, Australia, testing nearly 14,590 hours of EEG data across nine years. Our system prospectively labelled RPAH epilepsy ward data and subsequently reviewed by two neurologists and three certified EEG specialists. Our clinical result shows the proposed method achieves a 92.19% detection rate for an average time of 7.62 mins per 24 hrs of recorded 18-channel EEG. A human expert usually requires about 2 hrs of reviewing and labelling per any 24 hrs of recorded EEG and is often assisted by a wide range of auxiliary data such as patient, carer, or nurse inputs. In this prospective analysis, we consider humans' role as an expert arbiter who confirms to reject each alarm raised by our system. We achieved an average of 56 false alarms per 24 hrs.
Epileptic Seizure Classification with Symmetric and Hybrid Bilinear Models
Jan 15, 2020



Abstract:Epilepsy affects nearly 1% of the global population, of which two thirds can be treated by anti-epileptic drugs and a much lower percentage by surgery. Diagnostic procedures for epilepsy and monitoring are highly specialized and labour-intensive. The accuracy of the diagnosis is also complicated by overlapping medical symptoms, varying levels of experience and inter-observer variability among clinical professions. This paper proposes a novel hybrid bilinear deep learning network with an application in the clinical procedures of epilepsy classification diagnosis, where the use of surface electroencephalogram (sEEG) and audiovisual monitoring is standard practice. Hybrid bilinear models based on two types of feature extractors, namely Convolutional Neural Networks (CNNs) and Recurrent Neural Networks (RNNs), are trained using Short-Time Fourier Transform (STFT) of one-second sEEG. In the proposed hybrid models, CNNs extract spatio-temporal patterns, while RNNs focus on the characteristics of temporal dynamics in relatively longer intervals given the same input data. Second-order features, based on interactions between these spatio-temporal features are further explored by bilinear pooling and used for epilepsy classification. Our proposed methods obtain an F1-score of 97.4% on the Temple University Hospital Seizure Corpus and 97.2% on the EPILEPSIAE dataset, comparing favourably to existing benchmarks for sEEG-based seizure type classification. The open-source implementation of this study is available at https://github.com/NeuroSyd/Epileptic-Seizure-Classification
Machine Learning Cryptanalysis of a Quantum Random Number Generator
May 13, 2019



Abstract:Random number generators (RNGs) that are crucial for cryptographic applications have been the subject of adversarial attacks. These attacks exploit environmental information to predict generated random numbers that are supposed to be truly random and unpredictable. Though quantum random number generators (QRNGs) are based on the intrinsic indeterministic nature of quantum properties, the presence of classical noise in the measurement process compromises the integrity of a QRNG. In this paper, we develop a predictive machine learning (ML) analysis to investigate the impact of deterministic classical noise in different stages of an optical continuous variable QRNG. Our ML model successfully detects inherent correlations when the deterministic noise sources are prominent. After appropriate filtering and randomness extraction processes are introduced, our QRNG system, in turn, demonstrates its robustness against ML. We further demonstrate the robustness of our ML approach by applying it to uniformly distributed random numbers from the QRNG and a congruential RNG. Hence, our result shows that ML has potentials in benchmarking the quality of RNG devices.
Semi-supervised Seizure Prediction with Generative Adversarial Networks
Jun 20, 2018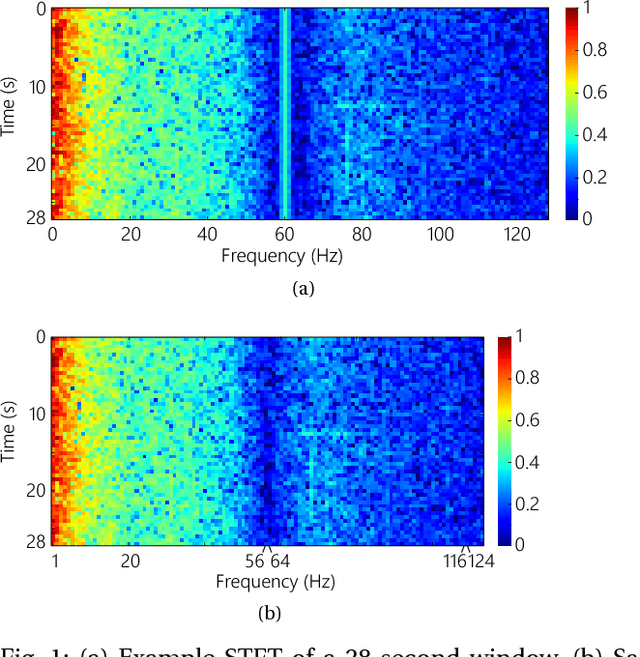
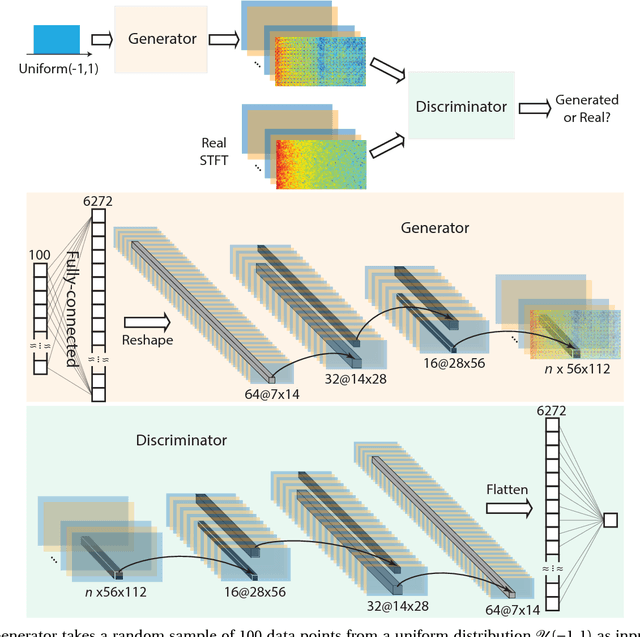
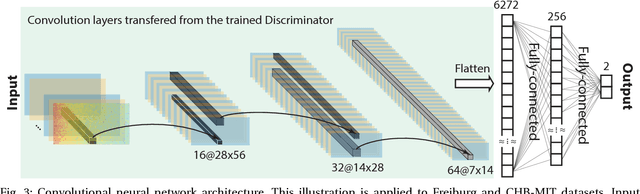
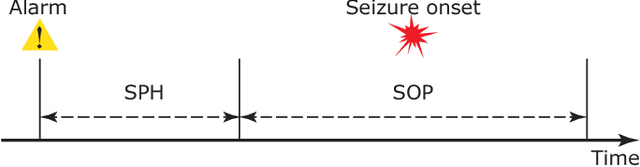
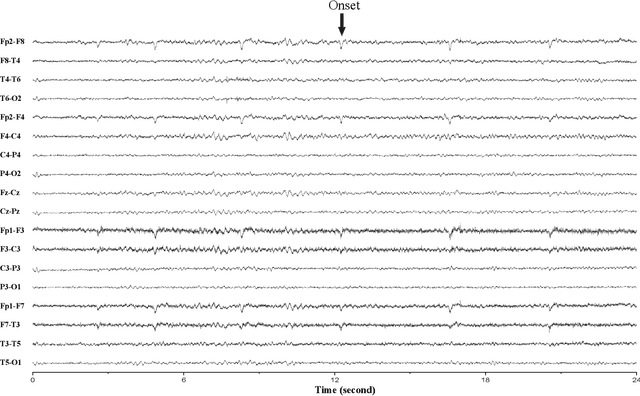
Abstract:In this article, we propose an approach that can make use of not only labeled EEG signals but also the unlabeled ones which is more accessible. We also suggest the use of data fusion to further improve the seizure prediction accuracy. Data fusion in our vision includes EEG signals, cardiogram signals, body temperature and time. We use the short-time Fourier transform on 28-s EEG windows as a pre-processing step. A generative adversarial network (GAN) is trained in an unsupervised manner where information of seizure onset is disregarded. The trained Discriminator of the GAN is then used as feature extractor. Features generated by the feature extractor are classified by two fully-connected layers (can be replaced by any classifier) for the labeled EEG signals. This semi-supervised seizure prediction method achieves area under the operating characteristic curve (AUC) of 77.68% and 75.47% for the CHBMIT scalp EEG dataset and the Freiburg Hospital intracranial EEG dataset, respectively. Unsupervised training without the need of labeling is important because not only it can be performed in real-time during EEG signal recording, but also it does not require feature engineering effort for each patient.
A Generalised Seizure Prediction with Convolutional Neural Networks for Intracranial and Scalp Electroencephalogram Data Analysis
Dec 06, 2017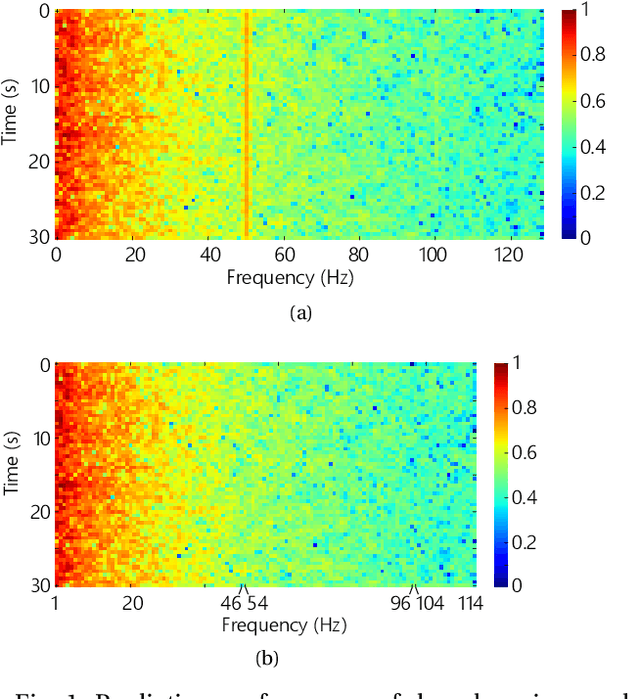
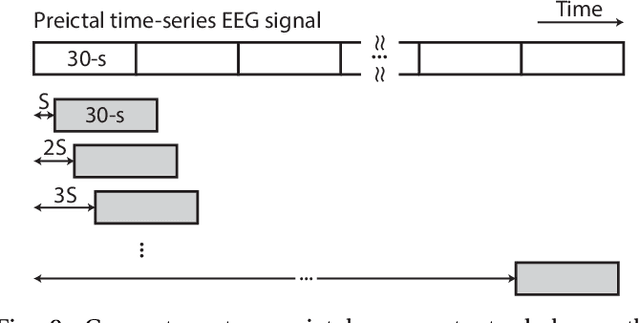
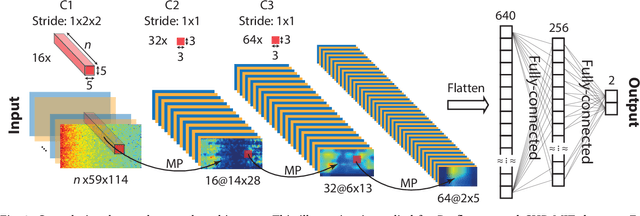

Abstract:Seizure prediction has attracted a growing attention as one of the most challenging predictive data analysis efforts in order to improve the life of patients living with drug-resistant epilepsy and tonic seizures. Many outstanding works have been reporting great results in providing a sensible indirect (warning systems) or direct (interactive neural-stimulation) control over refractory seizures, some of which achieved high performance. However, many works put heavily handcraft feature extraction and/or carefully tailored feature engineering to each patient to achieve very high sensitivity and low false prediction rate for a particular dataset. This limits the benefit of their approaches if a different dataset is used. In this paper we apply Convolutional Neural Networks (CNNs) on different intracranial and scalp electroencephalogram (EEG) datasets and proposed a generalized retrospective and patient-specific seizure prediction method. We use Short-Time Fourier Transform (STFT) on 30-second EEG windows with 50% overlapping to extract information in both frequency and time domains. A standardization step is then applied on STFT components across the whole frequency range to prevent high frequencies features being influenced by those at lower frequencies. A convolutional neural network model is used for both feature extraction and classification to separate preictal segments from interictal ones. The proposed approach achieves sensitivity of 81.4%, 81.2%, 82.3% and false prediction rate (FPR) of 0.06/h, 0.16/h, 0.22/h on Freiburg Hospital intracranial EEG (iEEG) dataset, Children's Hospital of Boston-MIT scalp EEG (sEEG) dataset, and Kaggle American Epilepsy Society Seizure Prediction Challenge's dataset, respectively. Our prediction method is also statistically better than an unspecific random predictor for most of patients in all three datasets.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge