Mohammad Mamouei
Clinical outcome prediction under hypothetical interventions -- a representation learning framework for counterfactual reasoning
May 15, 2022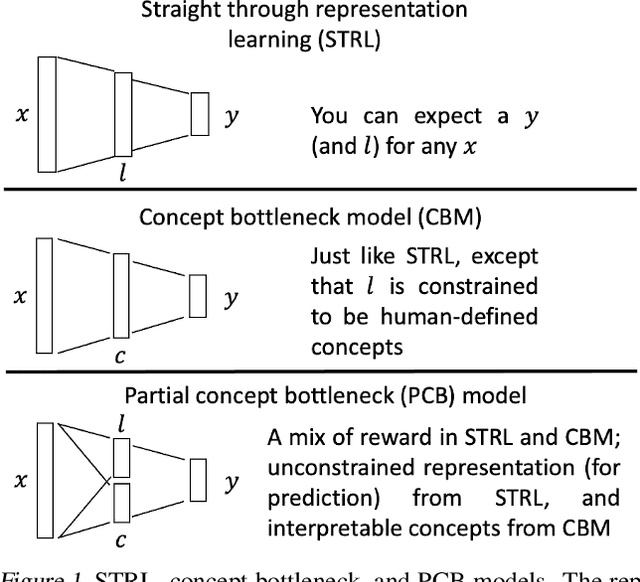
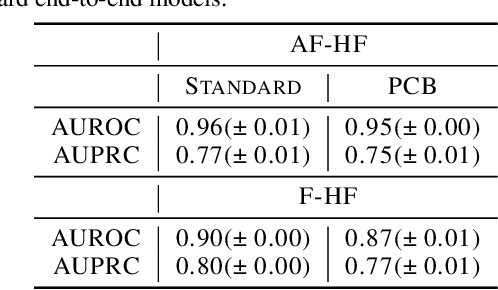
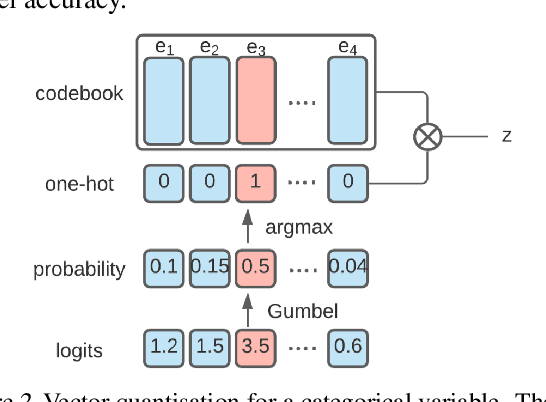
Abstract:Most machine learning (ML) models are developed for prediction only; offering no option for causal interpretation of their predictions or parameters/properties. This can hamper the health systems' ability to employ ML models in clinical decision-making processes, where the need and desire for predicting outcomes under hypothetical investigations (i.e., counterfactual reasoning/explanation) is high. In this research, we introduce a new representation learning framework (i.e., partial concept bottleneck), which considers the provision of counterfactual explanations as an embedded property of the risk model. Despite architectural changes necessary for jointly optimising for prediction accuracy and counterfactual reasoning, the accuracy of our approach is comparable to prediction-only models. Our results suggest that our proposed framework has the potential to help researchers and clinicians improve personalised care (e.g., by investigating the hypothetical differential effects of interventions)
Targeted-BEHRT: Deep learning for observational causal inference on longitudinal electronic health records
Feb 07, 2022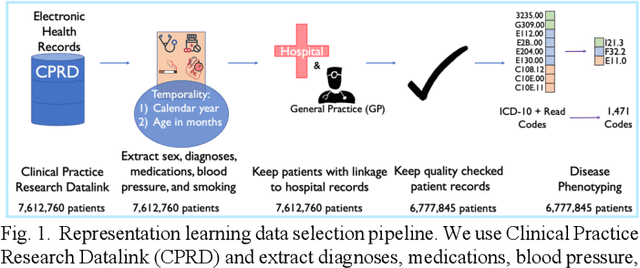
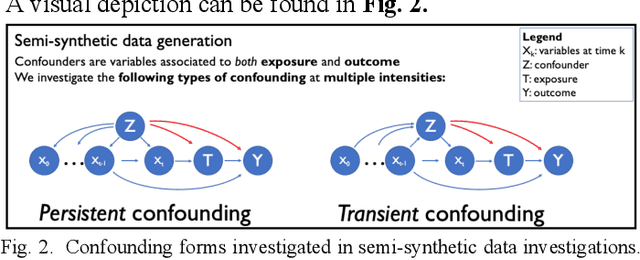
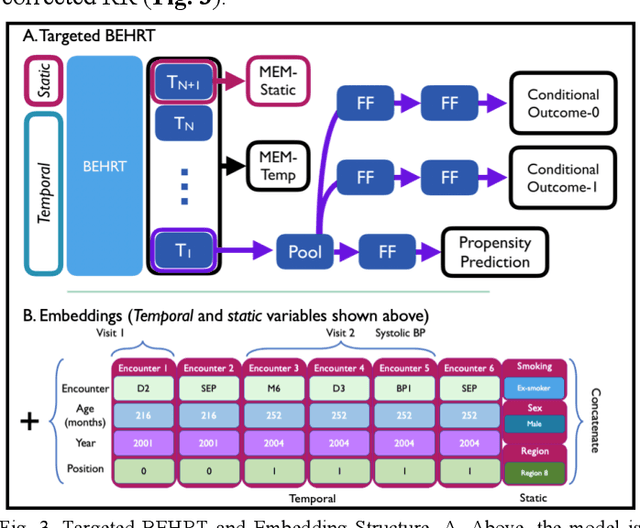
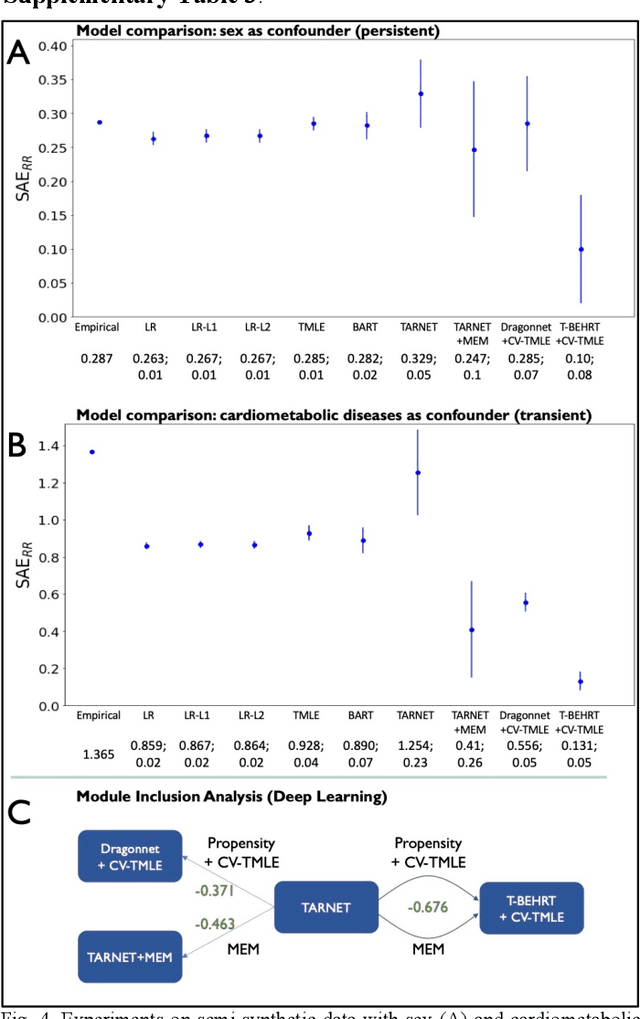
Abstract:Observational causal inference is useful for decision making in medicine when randomized clinical trials (RCT) are infeasible or non generalizable. However, traditional approaches fail to deliver unconfounded causal conclusions in practice. The rise of "doubly robust" non-parametric tools coupled with the growth of deep learning for capturing rich representations of multimodal data, offers a unique opportunity to develop and test such models for causal inference on comprehensive electronic health records (EHR). In this paper, we investigate causal modelling of an RCT-established null causal association: the effect of antihypertensive use on incident cancer risk. We develop a dataset for our observational study and a Transformer-based model, Targeted BEHRT coupled with doubly robust estimation, we estimate average risk ratio (RR). We compare our model to benchmark statistical and deep learning models for causal inference in multiple experiments on semi-synthetic derivations of our dataset with various types and intensities of confounding. In order to further test the reliability of our approach, we test our model on situations of limited data. We find that our model provides more accurate estimates of RR (least sum absolute error from ground truth) compared to benchmarks for risk ratio estimation on high-dimensional EHR across experiments. Finally, we apply our model to investigate the original case study: antihypertensives' effect on cancer and demonstrate that our model generally captures the validated null association.
Transfer Learning in Electronic Health Records through Clinical Concept Embedding
Jul 27, 2021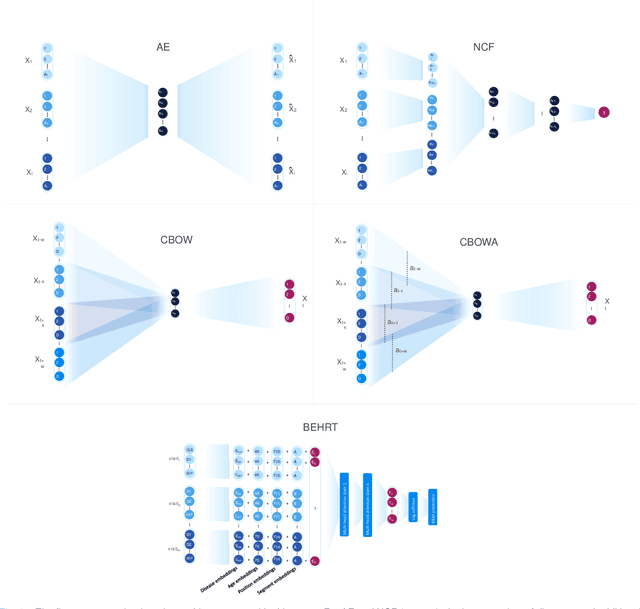
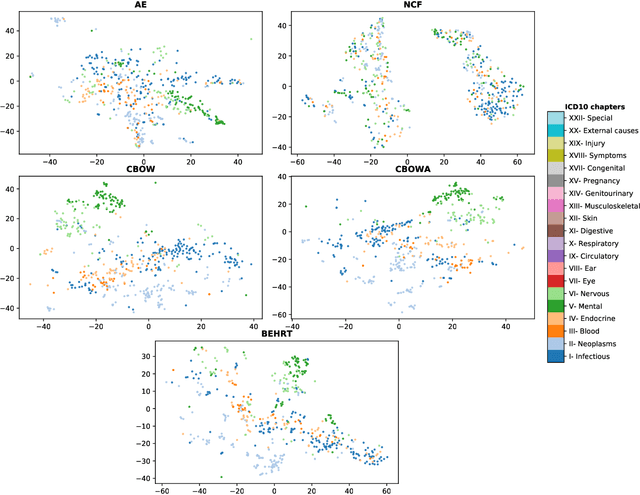
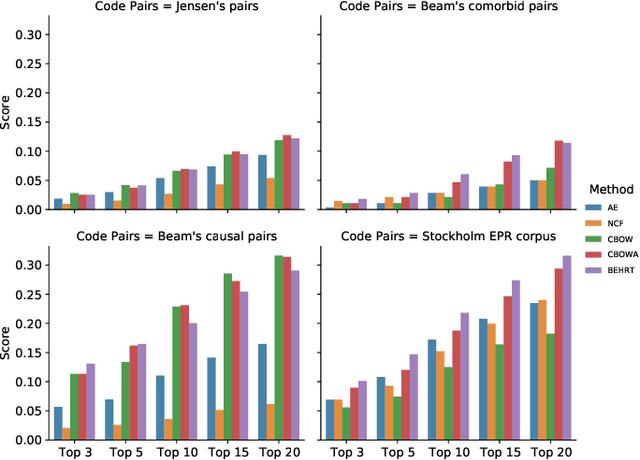
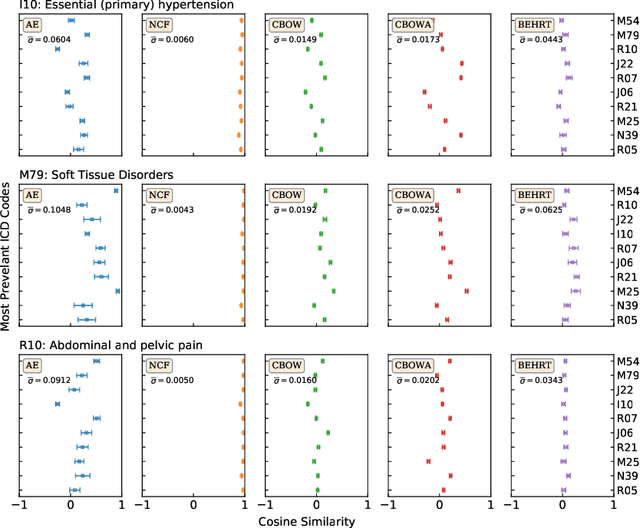
Abstract:Deep learning models have shown tremendous potential in learning representations, which are able to capture some key properties of the data. This makes them great candidates for transfer learning: Exploiting commonalities between different learning tasks to transfer knowledge from one task to another. Electronic health records (EHR) research is one of the domains that has witnessed a growing number of deep learning techniques employed for learning clinically-meaningful representations of medical concepts (such as diseases and medications). Despite this growth, the approaches to benchmark and assess such learned representations (or, embeddings) is under-investigated; this can be a big issue when such embeddings are shared to facilitate transfer learning. In this study, we aim to (1) train some of the most prominent disease embedding techniques on a comprehensive EHR data from 3.1 million patients, (2) employ qualitative and quantitative evaluation techniques to assess these embeddings, and (3) provide pre-trained disease embeddings for transfer learning. This study can be the first comprehensive approach for clinical concept embedding evaluation and can be applied to any embedding techniques and for any EHR concept.
Hi-BEHRT: Hierarchical Transformer-based model for accurate prediction of clinical events using multimodal longitudinal electronic health records
Jun 21, 2021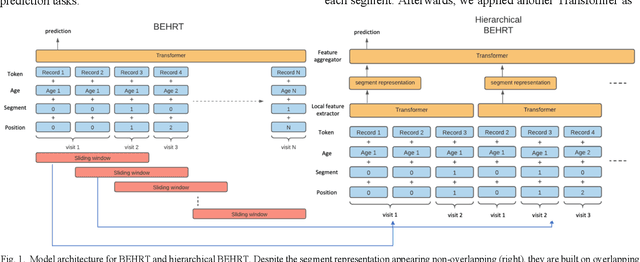
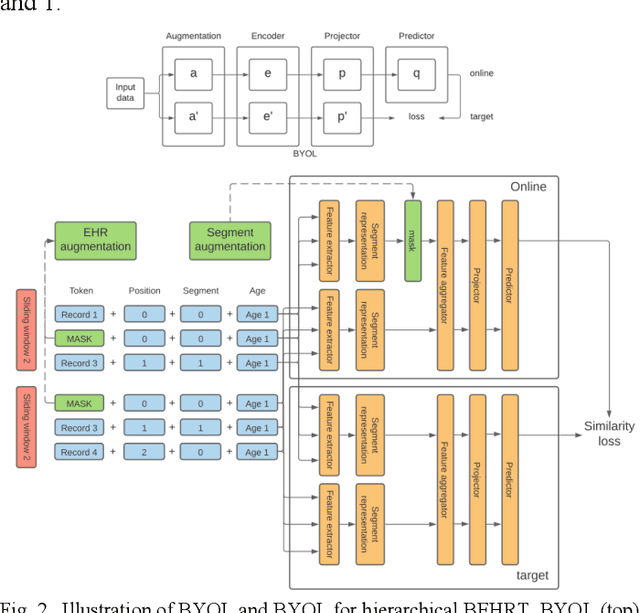
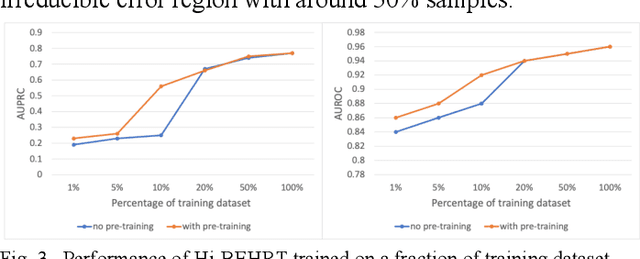
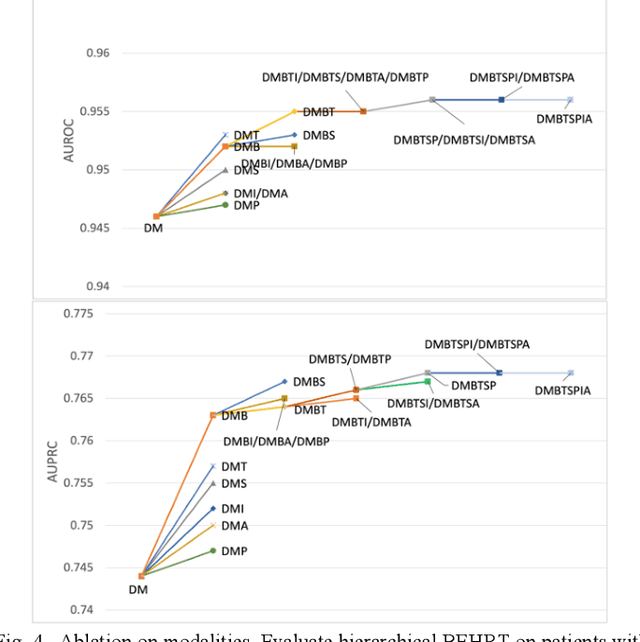
Abstract:Electronic health records represent a holistic overview of patients' trajectories. Their increasing availability has fueled new hopes to leverage them and develop accurate risk prediction models for a wide range of diseases. Given the complex interrelationships of medical records and patient outcomes, deep learning models have shown clear merits in achieving this goal. However, a key limitation of these models remains their capacity in processing long sequences. Capturing the whole history of medical encounters is expected to lead to more accurate predictions, but the inclusion of records collected for decades and from multiple resources can inevitably exceed the receptive field of the existing deep learning architectures. This can result in missing crucial, long-term dependencies. To address this gap, we present Hi-BEHRT, a hierarchical Transformer-based model that can significantly expand the receptive field of Transformers and extract associations from much longer sequences. Using a multimodal large-scale linked longitudinal electronic health records, the Hi-BEHRT exceeds the state-of-the-art BEHRT 1% to 5% for area under the receiver operating characteristic (AUROC) curve and 3% to 6% for area under the precision recall (AUPRC) curve on average, and 3% to 6% (AUROC) and 3% to 11% (AUPRC) for patients with long medical history for 5-year heart failure, diabetes, chronic kidney disease, and stroke risk prediction. Additionally, because pretraining for hierarchical Transformer is not well-established, we provide an effective end-to-end contrastive pre-training strategy for Hi-BEHRT using EHR, improving its transferability on predicting clinical events with relatively small training dataset.
Risk factor identification for incident heart failure using neural network distillation and variable selection
Mar 01, 2021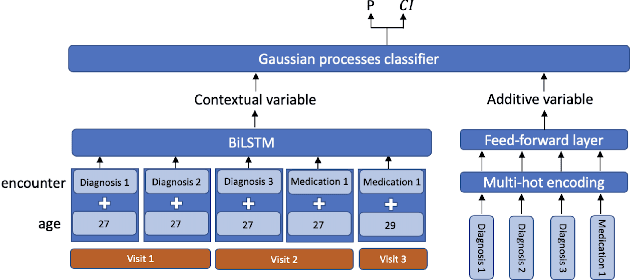
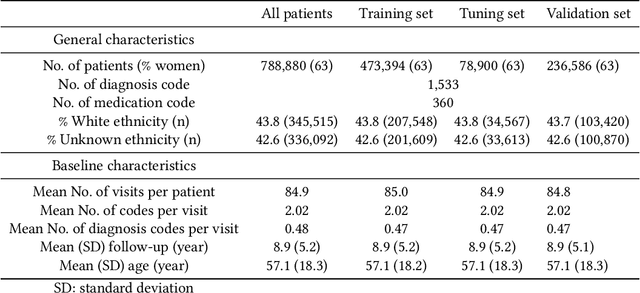
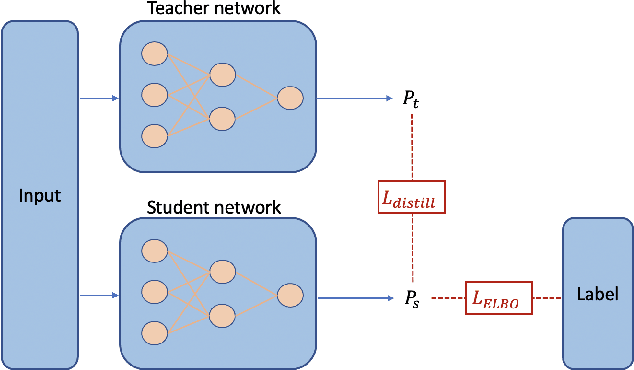
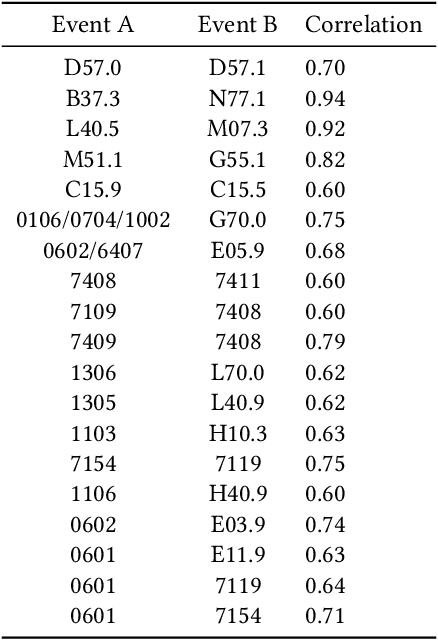
Abstract:Recent evidence shows that deep learning models trained on electronic health records from millions of patients can deliver substantially more accurate predictions of risk compared to their statistical counterparts. While this provides an important opportunity for improving clinical decision-making, the lack of interpretability is a major barrier to the incorporation of these black-box models in routine care, limiting their trustworthiness and preventing further hypothesis-testing investigations. In this study, we propose two methods, namely, model distillation and variable selection, to untangle hidden patterns learned by an established deep learning model (BEHRT) for risk association identification. Due to the clinical importance and diversity of heart failure as a phenotype, it was used to showcase the merits of the proposed methods. A cohort with 788,880 (8.3% incident heart failure) patients was considered for the study. Model distillation identified 598 and 379 diseases that were associated and dissociated with heart failure at the population level, respectively. While the associations were broadly consistent with prior knowledge, our method also highlighted several less appreciated links that are worth further investigation. In addition to these important population-level insights, we developed an approach to individual-level interpretation to take account of varying manifestation of heart failure in clinical practice. This was achieved through variable selection by detecting a minimal set of encounters that can maximally preserve the accuracy of prediction for individuals. Our proposed work provides a discovery-enabling tool to identify risk factors in both population and individual levels from a data-driven perspective. This helps to generate new hypotheses and guides further investigations on causal links.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge