Michael Porter
The intersection of video capsule endoscopy and artificial intelligence: addressing unique challenges using machine learning
Aug 24, 2023



Abstract:Introduction: Technical burdens and time-intensive review processes limit the practical utility of video capsule endoscopy (VCE). Artificial intelligence (AI) is poised to address these limitations, but the intersection of AI and VCE reveals challenges that must first be overcome. We identified five challenges to address. Challenge #1: VCE data are stochastic and contains significant artifact. Challenge #2: VCE interpretation is cost-intensive. Challenge #3: VCE data are inherently imbalanced. Challenge #4: Existing VCE AIMLT are computationally cumbersome. Challenge #5: Clinicians are hesitant to accept AIMLT that cannot explain their process. Methods: An anatomic landmark detection model was used to test the application of convolutional neural networks (CNNs) to the task of classifying VCE data. We also created a tool that assists in expert annotation of VCE data. We then created more elaborate models using different approaches including a multi-frame approach, a CNN based on graph representation, and a few-shot approach based on meta-learning. Results: When used on full-length VCE footage, CNNs accurately identified anatomic landmarks (99.1%), with gradient weighted-class activation mapping showing the parts of each frame that the CNN used to make its decision. The graph CNN with weakly supervised learning (accuracy 89.9%, sensitivity of 91.1%), the few-shot model (accuracy 90.8%, precision 91.4%, sensitivity 90.9%), and the multi-frame model (accuracy 97.5%, precision 91.5%, sensitivity 94.8%) performed well. Discussion: Each of these five challenges is addressed, in part, by one of our AI-based models. Our goal of producing high performance using lightweight models that aim to improve clinician confidence was achieved.
Graph Convolution Neural Network For Weakly Supervised Abnormality Localization In Long Capsule Endoscopy Videos
Oct 18, 2021
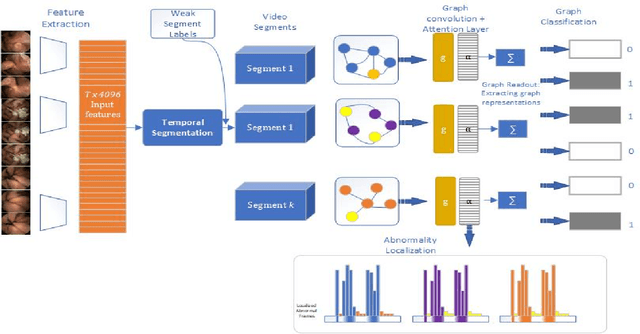
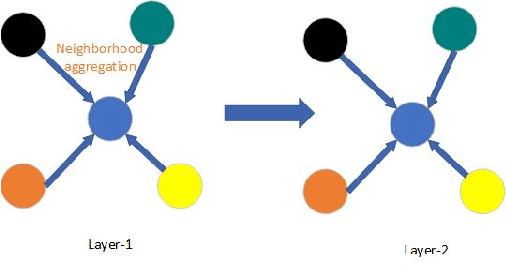
Abstract:Temporal activity localization in long videos is an important problem. The cost of obtaining frame level label for long Wireless Capsule Endoscopy (WCE) videos is prohibitive. In this paper, we propose an end-to-end temporal abnormality localization for long WCE videos using only weak video level labels. Physicians use Capsule Endoscopy (CE) as a non-surgical and non-invasive method to examine the entire digestive tract in order to diagnose diseases or abnormalities. While CE has revolutionized traditional endoscopy procedures, a single CE examination could last up to 8 hours generating as much as 100,000 frames. Physicians must review the entire video, frame-by-frame, in order to identify the frames capturing relevant abnormality. This, sometimes could be as few as just a single frame. Given this very high level of redundancy, analyzing long CE videos can be very tedious, time consuming and also error prone. This paper presents a novel multi-step method for an end-to-end localization of target frames capturing abnormalities of interest in the long video using only weak video labels. First we developed an automatic temporal segmentation using change point detection technique to temporally segment the video into uniform, homogeneous and identifiable segments. Then we employed Graph Convolutional Neural Network (GCNN) to learn a representation of each video segment. Using weak video segment labels, we trained our GCNN model to recognize each video segment as abnormal if it contains at least a single abnormal frame. Finally, leveraging the parameters of the trained GCNN model, we replaced the final layer of the network with a temporal pool layer to localize the relevant abnormal frames within each abnormal video segment. Our method achieved an accuracy of 89.9\% on the graph classification task and a specificity of 97.5\% on the abnormal frames localization task.
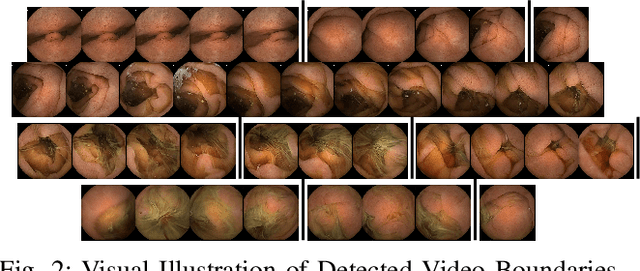
Unsupervised Shot Boundary Detection for Temporal Segmentation of Long Capsule Endoscopy Videos
Oct 18, 2021



Abstract:Physicians use Capsule Endoscopy (CE) as a non-invasive and non-surgical procedure to examine the entire gastrointestinal (GI) tract for diseases and abnormalities. A single CE examination could last between 8 to 11 hours generating up to 80,000 frames which is compiled as a video. Physicians have to review and analyze the entire video to identify abnormalities or diseases before making diagnosis. This review task can be very tedious, time consuming and prone to error. While only as little as a single frame may capture useful content that is relevant to the physicians' final diagnosis, frames covering the small bowel region alone could be as much as 50,000. To minimize physicians' review time and effort, this paper proposes a novel unsupervised and computationally efficient temporal segmentation method to automatically partition long CE videos into a homogeneous and identifiable video segments. However, the search for temporal boundaries in a long video using high dimensional frame-feature matrix is computationally prohibitive and impracticable for real clinical application. Therefore, leveraging both spatial and temporal information in the video, we first extracted high level frame features using a pretrained CNN model and then projected the high-dimensional frame-feature matrix to lower 1-dimensional embedding. Using this 1-dimensional sequence embedding, we applied the Pruned Exact Linear Time (PELT) algorithm to searched for temporal boundaries that indicates the transition points from normal to abnormal frames and vice-versa. We experimented with multiple real patients' CE videos and our model achieved an AUC of 66\% on multiple test videos against expert provided labels.
Lesion2Vec: Deep Metric Learning for Few-Shot Multiple Lesions Recognition in Wireless Capsule Endoscopy Video
Jan 15, 2021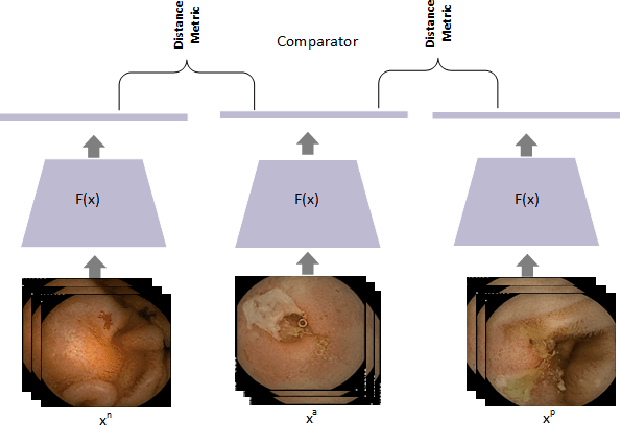
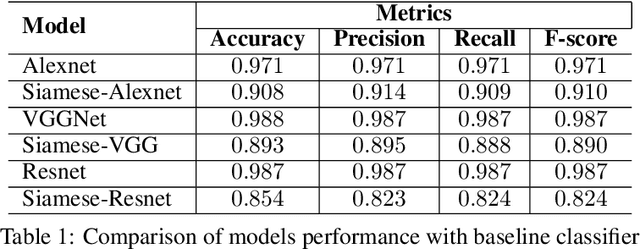
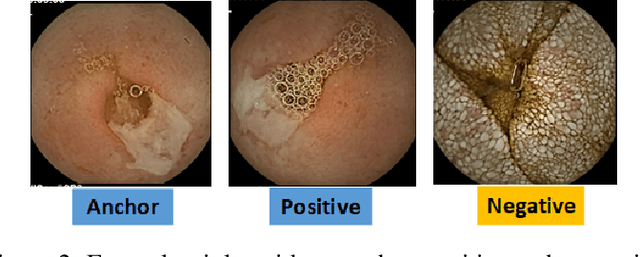
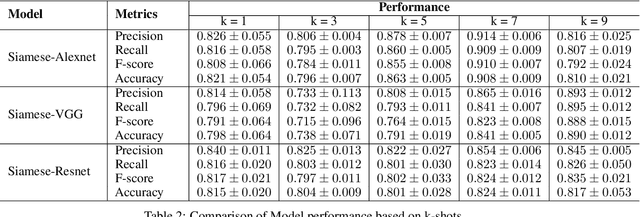
Abstract:Effective and rapid detection of lesions in the Gastrointestinal tract is critical to gastroenterologist's response to some life-threatening diseases. Wireless Capsule Endoscopy (WCE) has revolutionized traditional endoscopy procedure by allowing gastroenterologists visualize the entire GI tract non-invasively. Once the tiny capsule is swallowed, it sequentially capture images of the GI tract at about 2 to 6 frames per second (fps). A single video can last up to 8 hours producing between 30,000 to 100,000 images. Automating the detection of frames containing specific lesion in WCE video would relieve gastroenterologists the arduous task of reviewing the entire video before making diagnosis. While the WCE produces large volume of images, only about 5\% of the frames contain lesions that aid the diagnosis process. Convolutional Neural Network (CNN) based models have been very successful in various image classification tasks. However, they suffer excessive parameters, are sample inefficient and rely on very large amount of training data. Deploying a CNN classifier for lesion detection task will require time-to-time fine-tuning to generalize to any unforeseen category. In this paper, we propose a metric-based learning framework followed by a few-shot lesion recognition in WCE data. Metric-based learning is a meta-learning framework designed to establish similarity or dissimilarity between concepts while few-shot learning (FSL) aims to identify new concepts from only a small number of examples. We train a feature extractor to learn a representation for different small bowel lesions using metric-based learning. At the testing stage, the category of an unseen sample is predicted from only a few support examples, thereby allowing the model to generalize to a new category that has never been seen before. We demonstrated the efficacy of this method on real patient capsule endoscopy data.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge