Md Selim
Latent Diffusion Model for Medical Image Standardization and Enhancement
Oct 08, 2023



Abstract:Computed tomography (CT) serves as an effective tool for lung cancer screening, diagnosis, treatment, and prognosis, providing a rich source of features to quantify temporal and spatial tumor changes. Nonetheless, the diversity of CT scanners and customized acquisition protocols can introduce significant inconsistencies in texture features, even when assessing the same patient. This variability poses a fundamental challenge for subsequent research that relies on consistent image features. Existing CT image standardization models predominantly utilize GAN-based supervised or semi-supervised learning, but their performance remains limited. We present DiffusionCT, an innovative score-based DDPM model that operates in the latent space to transform disparate non-standard distributions into a standardized form. The architecture comprises a U-Net-based encoder-decoder, augmented by a DDPM model integrated at the bottleneck position. First, the encoder-decoder is trained independently, without embedding DDPM, to capture the latent representation of the input data. Second, the latent DDPM model is trained while keeping the encoder-decoder parameters fixed. Finally, the decoder uses the transformed latent representation to generate a standardized CT image, providing a more consistent basis for downstream analysis. Empirical tests on patient CT images indicate notable improvements in image standardization using DiffusionCT. Additionally, the model significantly reduces image noise in SPAD images, further validating the effectiveness of DiffusionCT for advanced imaging tasks.
DiffusionCT: Latent Diffusion Model for CT Image Standardization
Jan 20, 2023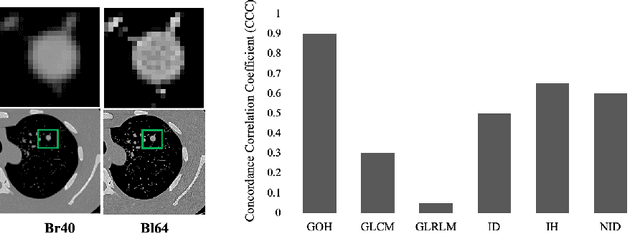
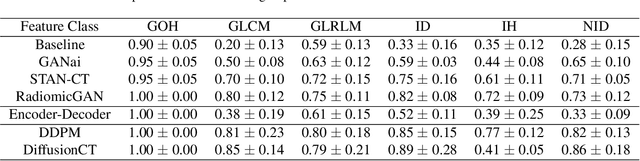
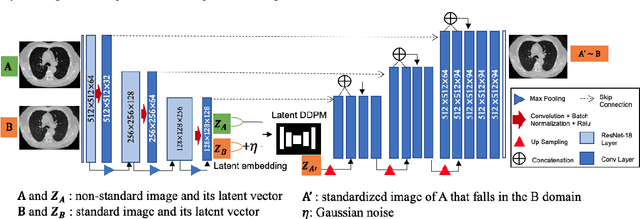
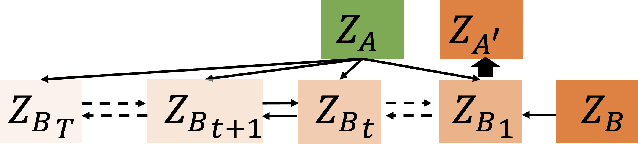
Abstract:Computed tomography (CT) imaging is a widely used modality for early lung cancer diagnosis, treatment, and prognosis. Features extracted from CT images are now accepted to quantify spatial and temporal variations in tumor architecture and function. However, CT images are often acquired using scanners from different vendors with customized acquisition standards, resulting in significantly different texture features even for the same patient, posing a fundamental challenge to downstream studies. Existing CT image harmonization models rely on supervised or semi-supervised techniques, with limited performance. In this paper, we have proposed a diffusion-based CT image standardization model called DiffusionCT which works on latent space by mapping latent distribution into a standard distribution. DiffusionCT incorporates an Unet-based encoder-decoder and a diffusion model embedded in its bottleneck part. The Unet first trained without the diffusion model to learn the latent representation of the input data. The diffusion model is trained in the next training phase. All the trained models work together on image standardization. The encoded representation outputted from the Unet encoder passes through the diffusion model, and the diffusion model maps the distribution in to target standard image domain. Finally, the decode takes that transformed latent representation to synthesize a standardized image. The experimental results show that DiffusionCT significantly improves the performance of the standardization task.
Cross-Vendor CT Image Data Harmonization Using CVH-CT
Oct 19, 2021



Abstract:While remarkable advances have been made in Computed Tomography (CT), most of the existing efforts focus on imaging enhancement while reducing radiation dose. How to harmonize CT image data captured using different scanners is vital in cross-center large-scale radiomics studies but remains the boundary to explore. Furthermore, the lack of paired training image problem makes it computationally challenging to adopt existing deep learning models. %developed for CT image standardization. %this problem more challenging. We propose a novel deep learning approach called CVH-CT for harmonizing CT images captured using scanners from different vendors. The generator of CVH-CT uses a self-attention mechanism to learn the scanner-related information. We also propose a VGG feature-based domain loss to effectively extract texture properties from unpaired image data to learn the scanner-based texture distributions. The experimental results show that CVH-CT is clearly better than the baselines because of the use of the proposed domain loss, and CVH-CT can effectively reduce the scanner-related variability in terms of radiomic features.
CT Image Harmonization for Enhancing Radiomics Studies
Jul 03, 2021



Abstract:While remarkable advances have been made in Computed Tomography (CT), capturing CT images with non-standardized protocols causes low reproducibility regarding radiomic features, forming a barrier on CT image analysis in a large scale. RadiomicGAN is developed to effectively mitigate the discrepancy caused by using non-standard reconstruction kernels. RadiomicGAN consists of hybrid neural blocks including both pre-trained and trainable layers adopted to learn radiomic feature distributions efficiently. A novel training approach, called Dynamic Window-based Training, has been developed to smoothly transform the pre-trained model to the medical imaging domain. Model performance evaluated using 1401 radiomic features show that RadiomicGAN clearly outperforms the state-of-art image standardization models.
STAN-CT: Standardizing CT Image using Generative Adversarial Network
Apr 02, 2020



Abstract:Computed tomography (CT) plays an important role in lung malignancy diagnostics and therapy assessment and facilitating precision medicine delivery. However, the use of personalized imaging protocols poses a challenge in large-scale cross-center CT image radiomic studies. We present an end-to-end solution called STAN-CT for CT image standardization and normalization, which effectively reduces discrepancies in image features caused by using different imaging protocols or using different CT scanners with the same imaging protocol. STAN-CT consists of two components: 1) a novel Generative Adversarial Networks (GAN) model that is capable of effectively learning the data distribution of a standard imaging protocol with only a few rounds of generator training, and 2) an automatic DICOM reconstruction pipeline with systematic image quality control that ensure the generation of high-quality standard DICOM images. Experimental results indicate that the training efficiency and model performance of STAN-CT have been significantly improved compared to the state-of-the-art CT image standardization and normalization algorithms.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge