Maria Anna Rapsomaniki
Hybrid quantum-classical graph neural networks for tumor classification in digital pathology
Oct 17, 2023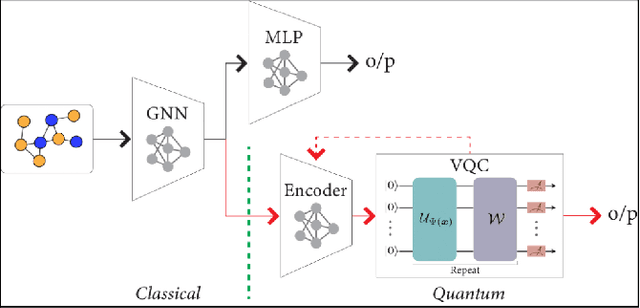
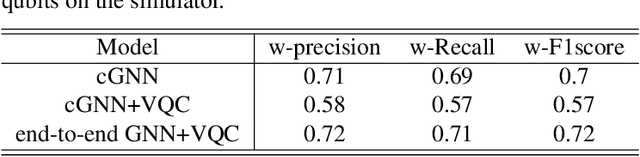
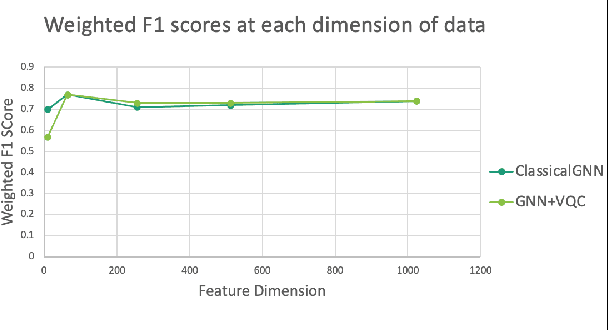
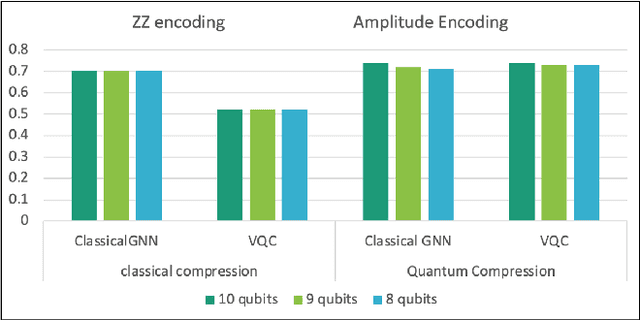
Abstract:Advances in classical machine learning and single-cell technologies have paved the way to understand interactions between disease cells and tumor microenvironments to accelerate therapeutic discovery. However, challenges in these machine learning methods and NP-hard problems in spatial Biology create an opportunity for quantum computing algorithms. We create a hybrid quantum-classical graph neural network (GNN) that combines GNN with a Variational Quantum Classifier (VQC) for classifying binary sub-tasks in breast cancer subtyping. We explore two variants of the same, the first with fixed pretrained GNN parameters and the second with end-to-end training of GNN+VQC. The results demonstrate that the hybrid quantum neural network (QNN) is at par with the state-of-the-art classical graph neural networks (GNN) in terms of weighted precision, recall and F1-score. We also show that by means of amplitude encoding, we can compress information in logarithmic number of qubits and attain better performance than using classical compression (which leads to information loss while keeping the number of qubits required constant in both regimes). Finally, we show that end-to-end training enables to improve over fixed GNN parameters and also slightly improves over vanilla GNN with same number of dimensions.
Inference of the three-dimensional chromatin structure and its temporal behavior
Nov 22, 2018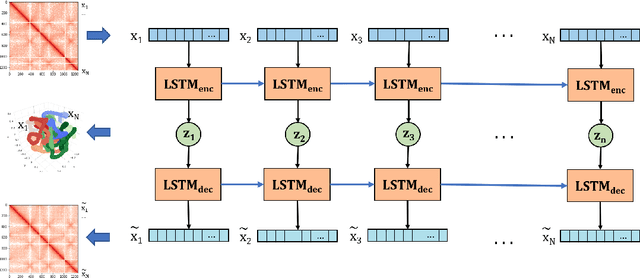
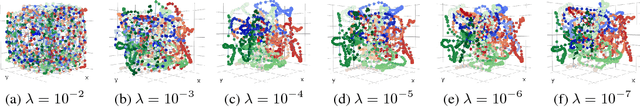
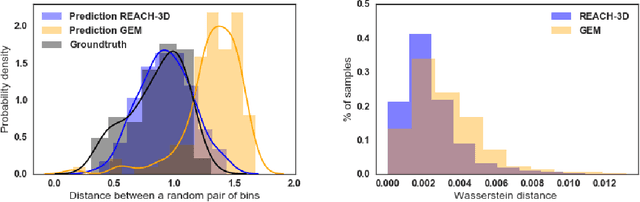
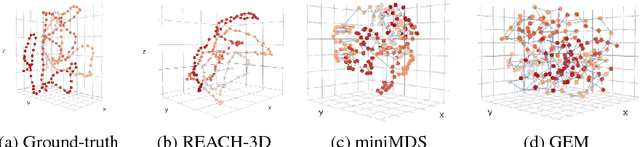
Abstract:Understanding the three-dimensional (3D) structure of the genome is essential for elucidating vital biological processes and their links to human disease. To determine how the genome folds within the nucleus, chromosome conformation capture methods such as HiC have recently been employed. However, computational methods that exploit the resulting high-throughput, high-resolution data are still suffering from important limitations. In this work, we explore the idea of manifold learning for the 3D chromatin structure inference and present a novel method, REcurrent Autoencoders for CHromatin 3D structure prediction (REACH-3D). Our framework employs autoencoders with recurrent neural units to reconstruct the chromatin structure. In comparison to existing methods, REACH-3D makes no transfer function assumption and permits dynamic analysis. Evaluating REACH-3D on synthetic data indicated high agreement with the ground truth. When tested on real experimental HiC data, REACH-3D recovered most faithfully the expected biological properties and obtained the highest correlation coefficient with microscopy measurements. Last, REACH-3D was applied to dynamic HiC data, where it successfully modeled chromatin conformation during the cell cycle.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge