Malak Itani
Observer study-based evaluation of a stochastic and physics-based method to generate oncological PET images
Feb 11, 2021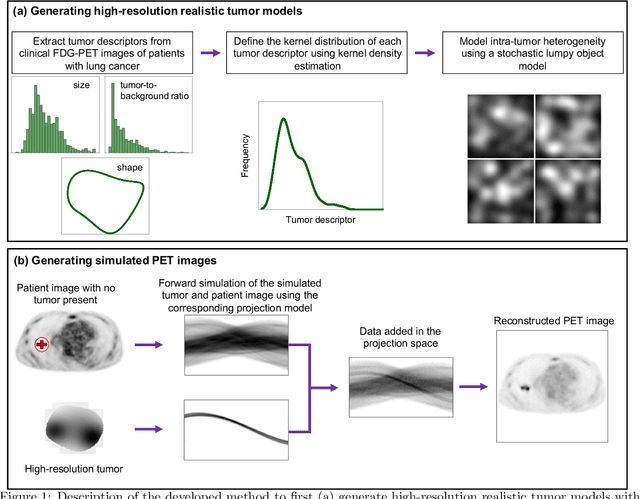


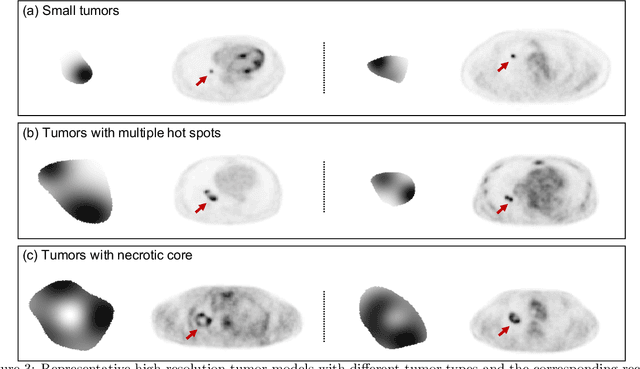
Abstract:Objective evaluation of new and improved methods for PET imaging requires access to images with ground truth, as can be obtained through simulation studies. However, for these studies to be clinically relevant, it is important that the simulated images are clinically realistic. In this study, we develop a stochastic and physics-based method to generate realistic oncological two-dimensional (2-D) PET images, where the ground-truth tumor properties are known. The developed method extends upon a previously proposed approach. The approach captures the observed variabilities in tumor properties from actual patient population. Further, we extend that approach to model intra-tumor heterogeneity using a lumpy object model. To quantitatively evaluate the clinical realism of the simulated images, we conducted a human-observer study. This was a two-alternative forced-choice (2AFC) study with trained readers (five PET physicians and one PET physicist). Our results showed that the readers had an average of ~ 50% accuracy in the 2AFC study. Further, the developed simulation method was able to generate wide varieties of clinically observed tumor types. These results provide evidence for the application of this method to 2-D PET imaging applications, and motivate development of this method to generate 3-D PET images.
An estimation-based method to segment PET images
Feb 29, 2020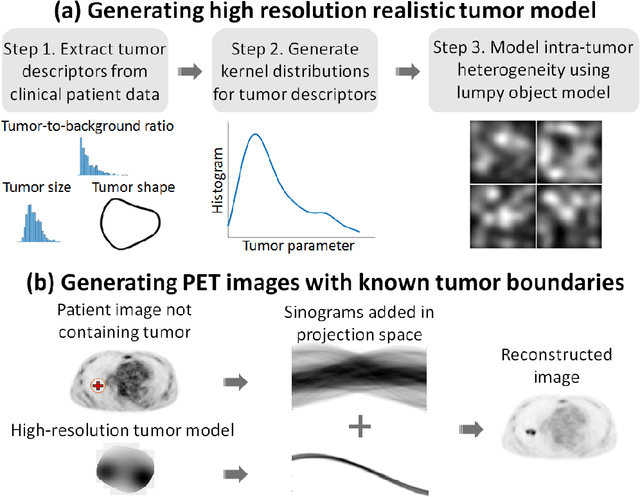
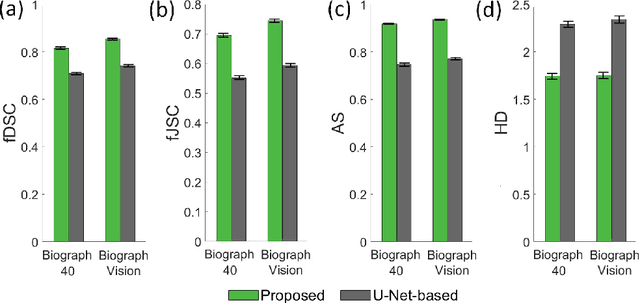


Abstract:Tumor segmentation in oncological PET images is challenging, a major reason being the partial-volume effects that arise from low system resolution and a finite pixel size. The latter results in pixels containing more than one region, also referred to as tissue-fraction effects. Conventional classification-based segmentation approaches are inherently limited in accounting for the tissue-fraction effects. To address this limitation, we pose the segmentation task as an estimation problem. We propose a Bayesian method that estimates the posterior mean of the tumorfraction area within each pixel and uses these estimates to define the segmented tumor boundary. The method was implemented using an autoencoder. Quantitative evaluation of the method was performed using realistic simulation studies conducted in the context of segmenting the primary tumor in PET images of patients with lung cancer. For these studies, a framework was developed to generate clinically realistic simulated PET images. Realism of these images was quantitatively confirmed using a two-alternative-forced-choice study by six trained readers with expertise in reading PET scans. The evaluation studies demonstrated that the proposed segmentation method was accurate, significantly outperformed widely used conventional methods on the tasks of tumor segmentation and estimation of tumor-fraction areas, was relatively insensitive to partial-volume effects, and reliably estimated the ground-truth tumor boundaries. Further, these results were obtained across different clinical-scanner configurations. This proof-of-concept study demonstrates the efficacy of an estimation-based approach to PET segmentation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge