Levy Chaves
Back to the Basics on Predicting Transfer Performance
May 30, 2024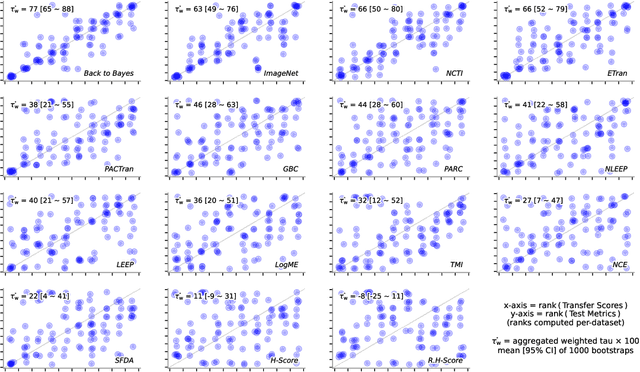
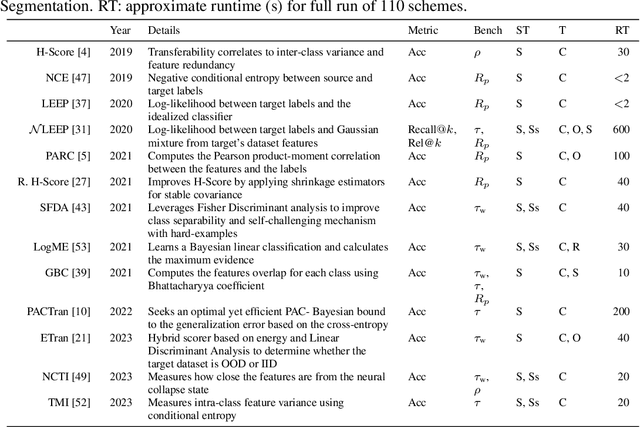
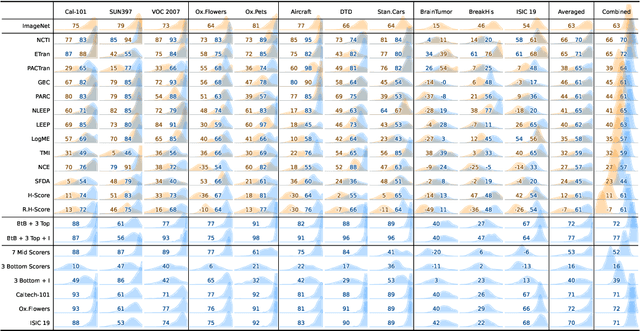
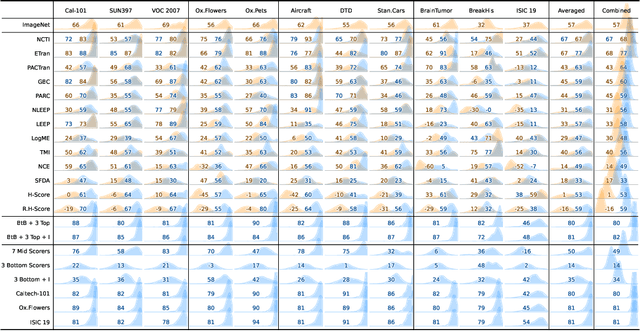
Abstract:In the evolving landscape of deep learning, selecting the best pre-trained models from a growing number of choices is a challenge. Transferability scorers propose alleviating this scenario, but their recent proliferation, ironically, poses the challenge of their own assessment. In this work, we propose both robust benchmark guidelines for transferability scorers, and a well-founded technique to combine multiple scorers, which we show consistently improves their results. We extensively evaluate 13 scorers from literature across 11 datasets, comprising generalist, fine-grained, and medical imaging datasets. We show that few scorers match the predictive performance of the simple raw metric of models on ImageNet, and that all predictors suffer on medical datasets. Our results highlight the potential of combining different information sources for reliably predicting transferability across varied domains.
Assessing the Generalizability of Deep Neural Networks-Based Models for Black Skin Lesions
Sep 30, 2023Abstract:Melanoma is the most severe type of skin cancer due to its ability to cause metastasis. It is more common in black people, often affecting acral regions: palms, soles, and nails. Deep neural networks have shown tremendous potential for improving clinical care and skin cancer diagnosis. Nevertheless, prevailing studies predominantly rely on datasets of white skin tones, neglecting to report diagnostic outcomes for diverse patient skin tones. In this work, we evaluate supervised and self-supervised models in skin lesion images extracted from acral regions commonly observed in black individuals. Also, we carefully curate a dataset containing skin lesions in acral regions and assess the datasets concerning the Fitzpatrick scale to verify performance on black skin. Our results expose the poor generalizability of these models, revealing their favorable performance for lesions on white skin. Neglecting to create diverse datasets, which necessitates the development of specialized models, is unacceptable. Deep neural networks have great potential to improve diagnosis, particularly for populations with limited access to dermatology. However, including black skin lesions is necessary to ensure these populations can access the benefits of inclusive technology.
The Performance of Transferability Metrics does not Translate to Medical Tasks
Aug 14, 2023

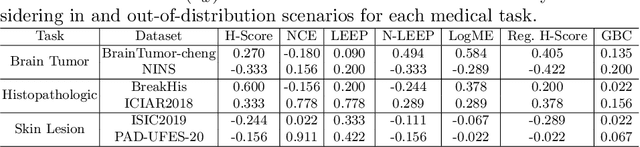
Abstract:Transfer learning boosts the performance of medical image analysis by enabling deep learning (DL) on small datasets through the knowledge acquired from large ones. As the number of DL architectures explodes, exhaustively attempting all candidates becomes unfeasible, motivating cheaper alternatives for choosing them. Transferability scoring methods emerge as an enticing solution, allowing to efficiently calculate a score that correlates with the architecture accuracy on any target dataset. However, since transferability scores have not been evaluated on medical datasets, their use in this context remains uncertain, preventing them from benefiting practitioners. We fill that gap in this work, thoroughly evaluating seven transferability scores in three medical applications, including out-of-distribution scenarios. Despite promising results in general-purpose datasets, our results show that no transferability score can reliably and consistently estimate target performance in medical contexts, inviting further work in that direction.
An Evaluation of Self-Supervised Pre-Training for Skin-Lesion Analysis
Jun 27, 2021
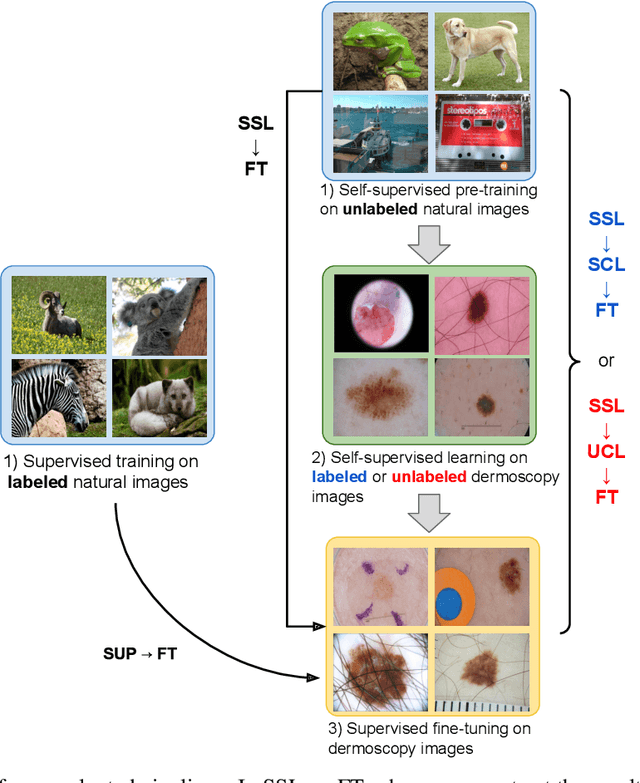
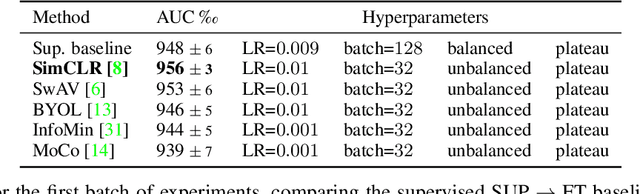
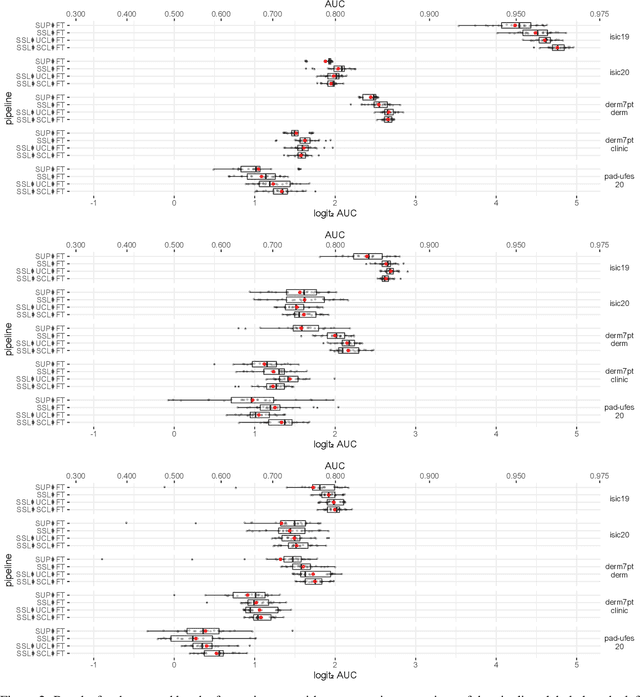
Abstract:Self-supervised pre-training appears as an advantageous alternative to supervised pre-trained for transfer learning. By synthesizing annotations on pretext tasks, self-supervision allows to pre-train models on large amounts of pseudo-labels before fine-tuning them on the target task. In this work, we assess self-supervision for the diagnosis of skin lesions, comparing three self-supervised pipelines to a challenging supervised baseline, on five test datasets comprising in- and out-of-distribution samples. Our results show that self-supervision is competitive both in improving accuracies and in reducing the variability of outcomes. Self-supervision proves particularly useful for low training data scenarios ($<1\,500$ and $<150$ samples), where its ability to stabilize the outcomes is essential to provide sound results.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge