Leonid Livshits
Sickle Cell Disease Severity Prediction from Percoll Gradient Images using Graph Convolutional Networks
Sep 11, 2021
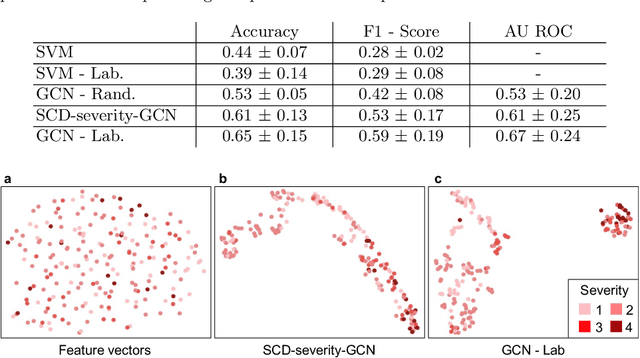


Abstract:Sickle cell disease (SCD) is a severe genetic hemoglobin disorder that results in premature destruction of red blood cells. Assessment of the severity of the disease is a challenging task in clinical routine since the causes of broad variance in SCD manifestation despite the common genetic cause remain unclear. Identification of the biomarkers that would predict the severity grade is of importance for prognosis and assessment of responsiveness of patients to therapy. Detection of the changes in red blood cell (RBC) density through separation of Percoll density gradient could be such marker as it allows to resolve intercellular differences and follow the most damaged dense cells prone to destruction and vaso-occlusion. Quantification of the images obtained from the distribution of RBCs in Percoll gradient and interpretation of the obtained is an important prerequisite for establishment of this approach. Here, we propose a novel approach combining a graph convolutional network, a convolutional neural network, fast Fourier transform, and recursive feature elimination to predict the severity of SCD directly from a Percoll image. Two important but expensive laboratory blood test parameters measurements are used for training the graph convolutional network. To make the model independent from such tests during prediction, the two parameters are estimated by a neural network from the Percoll image directly. On a cohort of 216 subjects, we achieve a prediction performance that is only slightly below an approach where the groundtruth laboratory measurements are used. Our proposed method is the first computational approach for the difficult task of SCD severity prediction. The two-step approach relies solely on inexpensive and simple blood analysis tools and can have a significant impact on the patients' survival in underdeveloped countries where access to medical instruments and doctors is limited
Fourier Transform of Percoll Gradients Boosts CNN Classification of Hereditary Hemolytic Anemias
Mar 17, 2021

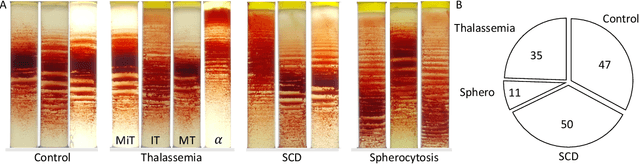
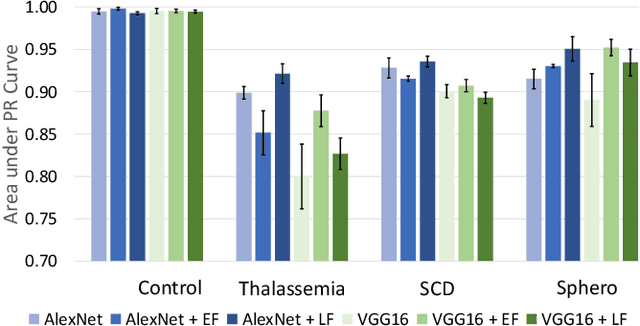
Abstract:Hereditary hemolytic anemias are genetic disorders that affect the shape and density of red blood cells. Genetic tests currently used to diagnose such anemias are expensive and unavailable in the majority of clinical labs. Here, we propose a method for identifying hereditary hemolytic anemias based on a standard biochemistry method, called Percoll gradient, obtained by centrifuging a patient's blood. Our hybrid approach consists on using spatial data-driven features, extracted with a convolutional neural network and spectral handcrafted features obtained from fast Fourier transform. We compare late and early feature fusion with AlexNet and VGG16 architectures. AlexNet with late fusion of spectral features performs better compared to other approaches. We achieved an average F1-score of 88% on different classes suggesting the possibility of diagnosing of hereditary hemolytic anemias from Percoll gradients. Finally, we utilize Grad-CAM to explore the spatial features used for classification.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge