Krishnakant V. Saboo
REMEDI: REinforcement learning-driven adaptive MEtabolism modeling of primary sclerosing cholangitis DIsease progression
Oct 02, 2023



Abstract:Primary sclerosing cholangitis (PSC) is a rare disease wherein altered bile acid metabolism contributes to sustained liver injury. This paper introduces REMEDI, a framework that captures bile acid dynamics and the body's adaptive response during PSC progression that can assist in exploring treatments. REMEDI merges a differential equation (DE)-based mechanistic model that describes bile acid metabolism with reinforcement learning (RL) to emulate the body's adaptations to PSC continuously. An objective of adaptation is to maintain homeostasis by regulating enzymes involved in bile acid metabolism. These enzymes correspond to the parameters of the DEs. REMEDI leverages RL to approximate adaptations in PSC, treating homeostasis as a reward signal and the adjustment of the DE parameters as the corresponding actions. On real-world data, REMEDI generated bile acid dynamics and parameter adjustments consistent with published findings. Also, our results support discussions in the literature that early administration of drugs that suppress bile acid synthesis may be effective in PSC treatment.
Reinforcement Learning based Disease Progression Model for Alzheimer's Disease
Jun 30, 2021
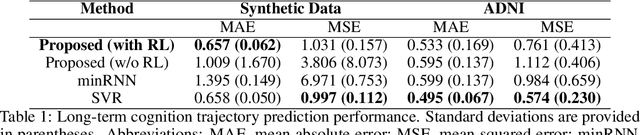
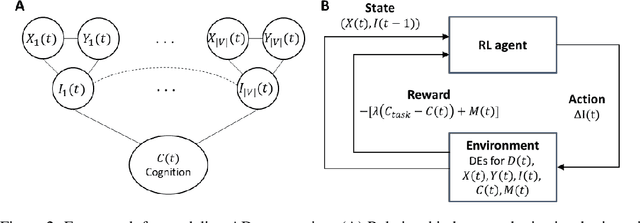
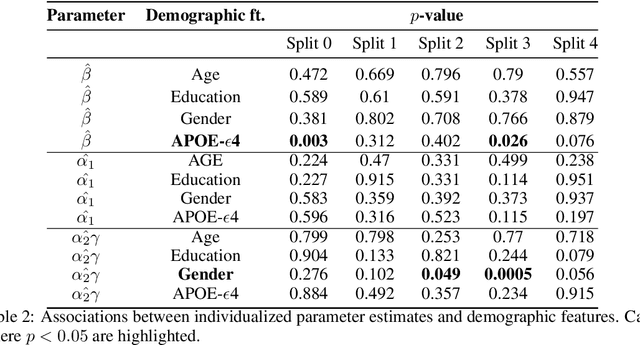
Abstract:We model Alzheimer's disease (AD) progression by combining differential equations (DEs) and reinforcement learning (RL) with domain knowledge. DEs provide relationships between some, but not all, factors relevant to AD. We assume that the missing relationships must satisfy general criteria about the working of the brain, for e.g., maximizing cognition while minimizing the cost of supporting cognition. This allows us to extract the missing relationships by using RL to optimize an objective (reward) function that captures the above criteria. We use our model consisting of DEs (as a simulator) and the trained RL agent to predict individualized 10-year AD progression using baseline (year 0) features on synthetic and real data. The model was comparable or better at predicting 10-year cognition trajectories than state-of-the-art learning-based models. Our interpretable model demonstrated, and provided insights into, "recovery/compensatory" processes that mitigate the effect of AD, even though those processes were not explicitly encoded in the model. Our framework combines DEs with RL for modelling AD progression and has broad applicability for understanding other neurological disorders.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge