Anirudh Choudhary
RACR-MIL: Weakly Supervised Skin Cancer Grading using Rank-Aware Contextual Reasoning on Whole Slide Images
Aug 29, 2023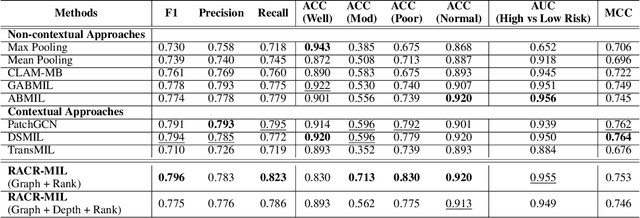
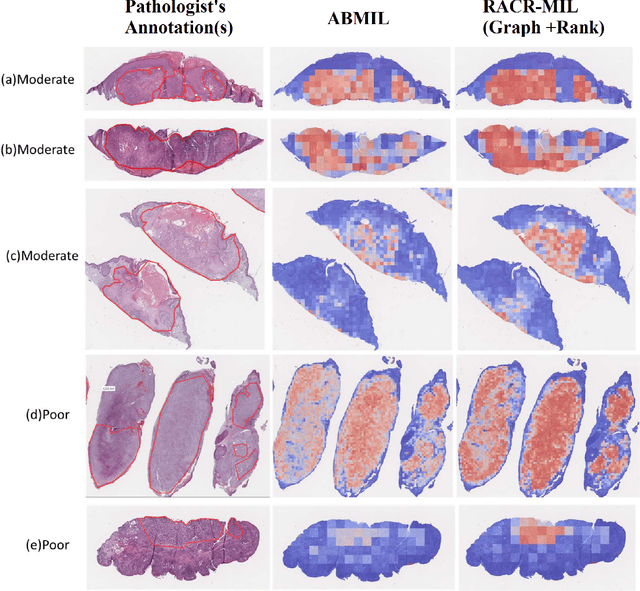
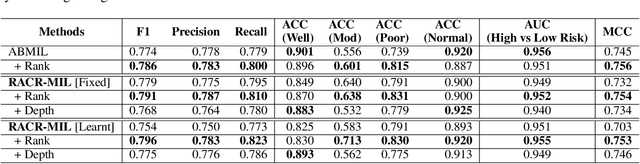
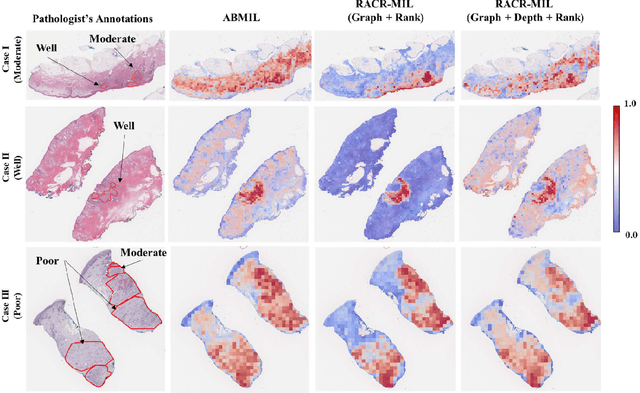
Abstract:Cutaneous squamous cell cancer (cSCC) is the second most common skin cancer in the US. It is diagnosed by manual multi-class tumor grading using a tissue whole slide image (WSI), which is subjective and suffers from inter-pathologist variability. We propose an automated weakly-supervised grading approach for cSCC WSIs that is trained using WSI-level grade and does not require fine-grained tumor annotations. The proposed model, RACR-MIL, transforms each WSI into a bag of tiled patches and leverages attention-based multiple-instance learning to assign a WSI-level grade. We propose three key innovations to address general as well as cSCC-specific challenges in tumor grading. First, we leverage spatial and semantic proximity to define a WSI graph that encodes both local and non-local dependencies between tumor regions and leverage graph attention convolution to derive contextual patch features. Second, we introduce a novel ordinal ranking constraint on the patch attention network to ensure that higher-grade tumor regions are assigned higher attention. Third, we use tumor depth as an auxiliary task to improve grade classification in a multitask learning framework. RACR-MIL achieves 2-9% improvement in grade classification over existing weakly-supervised approaches on a dataset of 718 cSCC tissue images and localizes the tumor better. The model achieves 5-20% higher accuracy in difficult-to-classify high-risk grade classes and is robust to class imbalance.
Reinforcement Learning based Disease Progression Model for Alzheimer's Disease
Jun 30, 2021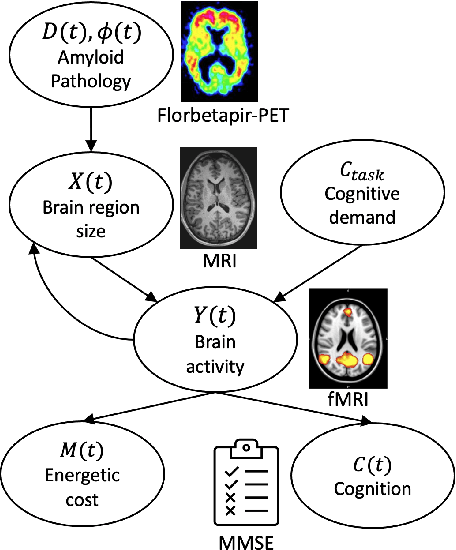
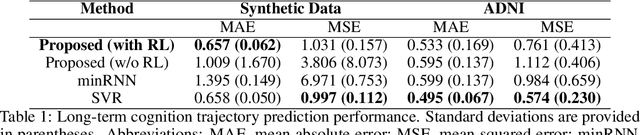
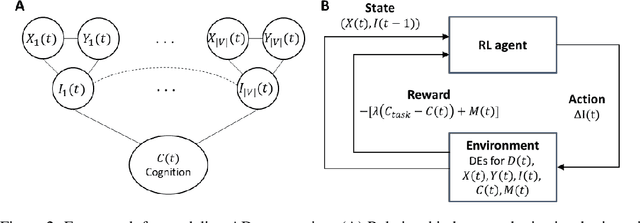
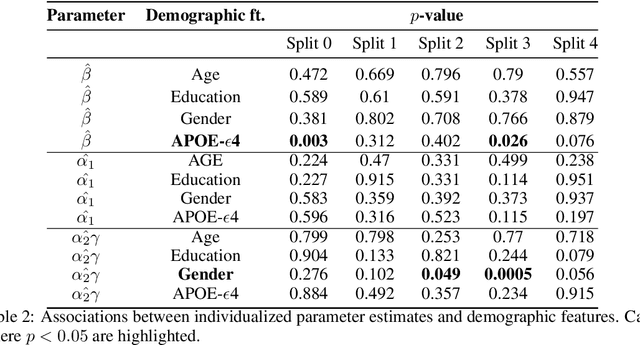
Abstract:We model Alzheimer's disease (AD) progression by combining differential equations (DEs) and reinforcement learning (RL) with domain knowledge. DEs provide relationships between some, but not all, factors relevant to AD. We assume that the missing relationships must satisfy general criteria about the working of the brain, for e.g., maximizing cognition while minimizing the cost of supporting cognition. This allows us to extract the missing relationships by using RL to optimize an objective (reward) function that captures the above criteria. We use our model consisting of DEs (as a simulator) and the trained RL agent to predict individualized 10-year AD progression using baseline (year 0) features on synthetic and real data. The model was comparable or better at predicting 10-year cognition trajectories than state-of-the-art learning-based models. Our interpretable model demonstrated, and provided insights into, "recovery/compensatory" processes that mitigate the effect of AD, even though those processes were not explicitly encoded in the model. Our framework combines DEs with RL for modelling AD progression and has broad applicability for understanding other neurological disorders.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge