Johanna Luitjens
Foundations of a Knee Joint Digital Twin from qMRI Biomarkers for Osteoarthritis and Knee Replacement
Jan 26, 2025Abstract:This study forms the basis of a digital twin system of the knee joint, using advanced quantitative MRI (qMRI) and machine learning to advance precision health in osteoarthritis (OA) management and knee replacement (KR) prediction. We combined deep learning-based segmentation of knee joint structures with dimensionality reduction to create an embedded feature space of imaging biomarkers. Through cross-sectional cohort analysis and statistical modeling, we identified specific biomarkers, including variations in cartilage thickness and medial meniscus shape, that are significantly associated with OA incidence and KR outcomes. Integrating these findings into a comprehensive framework represents a considerable step toward personalized knee-joint digital twins, which could enhance therapeutic strategies and inform clinical decision-making in rheumatological care. This versatile and reliable infrastructure has the potential to be extended to broader clinical applications in precision health.
The object detection method aids in image reconstruction evaluation and clinical interpretation of meniscal abnormalities
Jul 16, 2024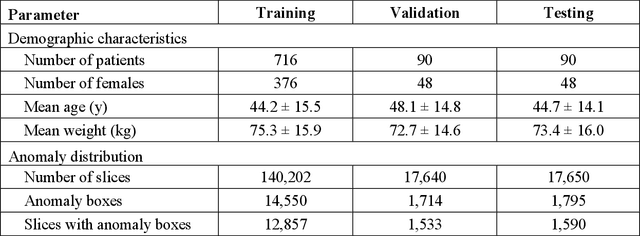
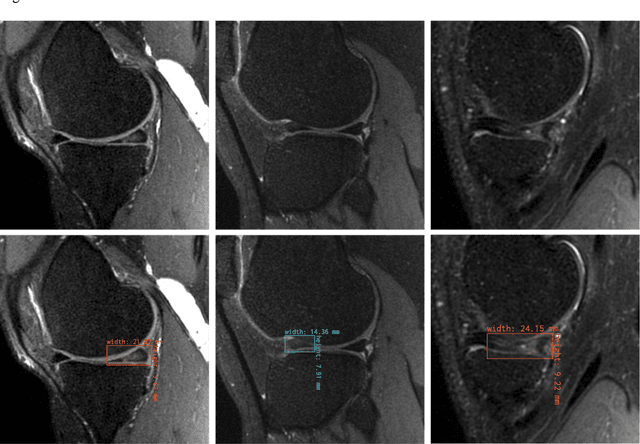
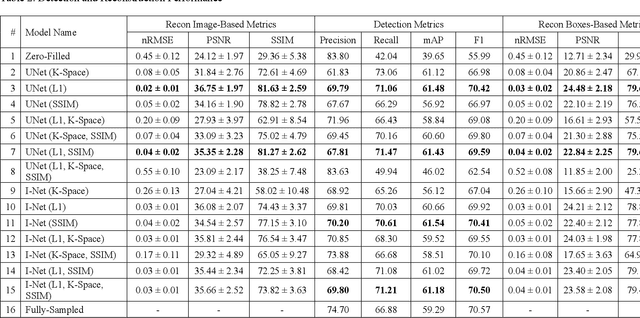
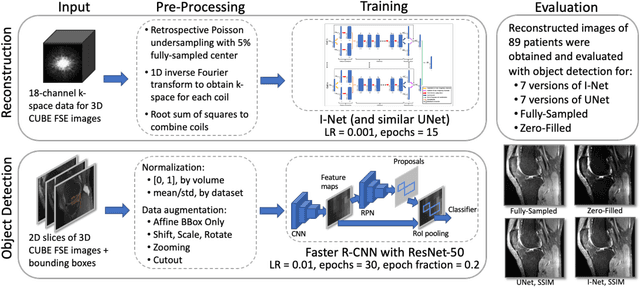
Abstract:This study investigates the relationship between deep learning (DL) image reconstruction quality and anomaly detection performance, and evaluates the efficacy of an artificial intelligence (AI) assistant in enhancing radiologists' interpretation of meniscal anomalies on reconstructed images. A retrospective study was conducted using an in-house reconstruction and anomaly detection pipeline to assess knee MR images from 896 patients. The original and 14 sets of DL-reconstructed images were evaluated using standard reconstruction and object detection metrics, alongside newly developed box-based reconstruction metrics. Two clinical radiologists reviewed a subset of 50 patients' images, both original and AI-assisted reconstructed, with subsequent assessment of their accuracy and performance characteristics. Results indicated that the structural similarity index (SSIM) showed a weaker correlation with anomaly detection metrics (mAP, r=0.64, p=0.01; F1 score, r=0.38, p=0.18), while box-based SSIM had a stronger association with detection performance (mAP, r=0.81, p<0.01; F1 score, r=0.65, p=0.01). Minor SSIM fluctuations did not affect detection outcomes, but significant changes reduced performance. Radiologists' AI-assisted evaluations demonstrated improved accuracy (86.0% without assistance vs. 88.3% with assistance, p<0.05) and interrater agreement (Cohen's kappa, 0.39 without assistance vs. 0.57 with assistance). An additional review led to the incorporation of 17 more lesions into the dataset. The proposed anomaly detection method shows promise in evaluating reconstruction algorithms for automated tasks and aiding radiologists in interpreting DL-reconstructed MR images.
Technical Note: Feasibility of translating 3.0T-trained Deep-Learning Segmentation Models Out-of-the-Box on Low-Field MRI 0.55T Knee-MRI of Healthy Controls
Oct 26, 2023Abstract:In the current study, our purpose is to evaluate the feasibility of applying deep learning (DL) enabled algorithms to quantify bilateral knee biomarkers in healthy controls scanned at 0.55T and compared with 3.0T. The current study assesses the performance of standard in-practice bone, and cartilage segmentation algorithms at 0.55T, both qualitatively and quantitatively, in terms of comparing segmentation performance, areas of improvement, and compartment-wise cartilage thickness values between 0.55T vs. 3.0T. Initial results demonstrate a usable to good technical feasibility of translating existing quantitative deep-learning-based image segmentation techniques, trained on 3.0T, out of 0.55T for knee MRI, in a multi-vendor acquisition environment. Especially in terms of segmenting cartilage compartments, the models perform almost equivalent to 3.0T in terms of Likert ranking. The 0.55T low-field sustainable and easy-to-install MRI, as demonstrated, thus, can be utilized for evaluating knee cartilage thickness and bone segmentations aided by established DL algorithms trained at higher-field strengths out-of-the-box initially. This could be utilized at the far-spread point-of-care locations with a lack of radiologists available to manually segment low-field images, at least till a decent base of low-field data pool is collated. With further fine-tuning with manual labeling of low-field data or utilizing synthesized higher SNR images from low-field images, OA biomarker quantification performance is potentially guaranteed to be further improved.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge