Joan Glaunès
How to Register a Live onto a Liver ? Partial Matching in the Space of Varifolds
Apr 12, 2022



Abstract:Partial shapes correspondences is a problem that often occurs in computer vision (occlusion, evolution in time...). In medical imaging, data may come from different modalities and be acquired under different conditions which leads to variations in shapes and topologies. In this paper we use an asymmetric data dissimilarity term applicable to various geometric shapes like sets of curves or surfaces, assessing the embedding of a shape into another one without relying on correspondences. It is designed as a data attachment for the Large Deformation Diffeomorphic Metric Mapping (LDDMM) framework, allowing to compute a meaningful deformation of one shape onto a subset of the other. We refine it in order to control the resulting non-rigid deformations and provide consistent deformations of the shapes along with their ambient space. We show that partial matching can be used for robust multi-modal liver registration between a Computed Tomography (CT) volume and a Cone Beam Computed Tomography (CBCT) volume. The 3D imaging of the patient CBCT at point of care that we call live is truncated while the CT pre-intervention provides a full visualization of the liver. The proposed method allows the truncated surfaces from CBCT to be aligned non-rigidly, yet realistically, with surfaces from CT with an average distance of 2.6mm(+/- 2.2). The generated deformations extend consistently to the liver volume, and are evaluated on points of interest for the physicians, with an average distance of 5.8mm (+/- 2.7) for vessels bifurcations and 5.13mm (+/- 2.5) for tumors landmarks. Such multi-modality volumes registrations would help the physicians in the perspective of navigating their tools in the patient's anatomy to locate structures that are hardly visible in the CBCT used during their procedures. Our code is available at https://github.com/plantonsanti/PartialMatchingVarifolds.
* 30 pages, 11 figures, Special Issue: Information Processing in Medical Imaging (IPMI) 2021, Accepted for publication at the Journal of Machine Learning for Biomedical Imaging (MELBA) https://www.melba-journal.org
A deep residual learning implementation of Metamorphosis
Feb 01, 2022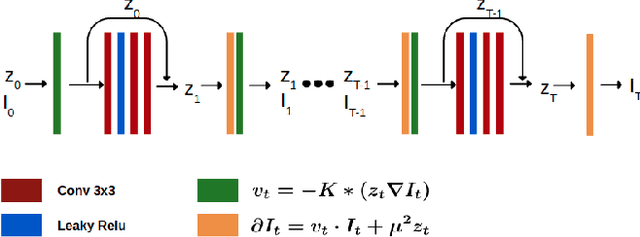
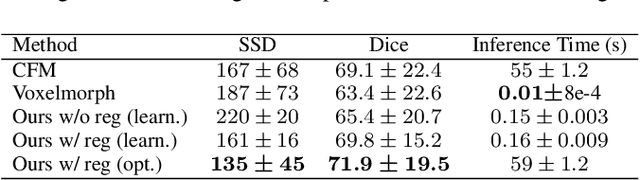
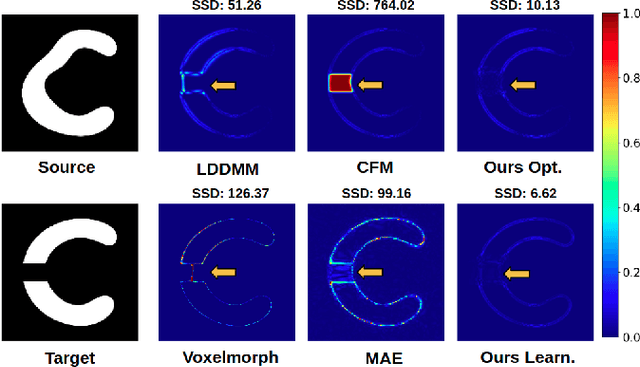
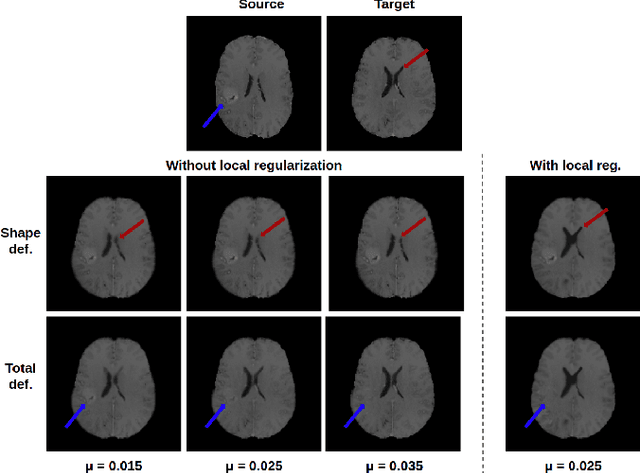
Abstract:In medical imaging, most of the image registration methods implicitly assume a one-to-one correspondence between the source and target images (i.e., diffeomorphism). However, this is not necessarily the case when dealing with pathological medical images (e.g., presence of a tumor, lesion, etc.). To cope with this issue, the Metamorphosis model has been proposed. It modifies both the shape and the appearance of an image to deal with the geometrical and topological differences. However, the high computational time and load have hampered its applications so far. Here, we propose a deep residual learning implementation of Metamorphosis that drastically reduces the computational time at inference. Furthermore, we also show that the proposed framework can easily integrate prior knowledge of the localization of topological changes (e.g., segmentation masks) that can act as spatial regularization to correctly disentangle appearance and shape changes. We test our method on the BraTS 2021 dataset, showing that it outperforms current state-of-the-art methods in the alignment of images with brain tumors.
Metamorphic image registration using a semi-Lagrangian scheme
Jun 16, 2021

Abstract:In this paper, we propose an implementation of both Large Deformation Diffeomorphic Metric Mapping (LDDMM) and Metamorphosis image registration using a semi-Lagrangian scheme for geodesic shooting. We propose to solve both problems as an inexact matching providing a single and unifying cost function. We demonstrate that for image registration the use of a semi-Lagrangian scheme is more stable than a standard Eulerian scheme. Our GPU implementation is based on PyTorch, which greatly simplifies and accelerates the computations thanks to its powerful automatic differentiation engine. It will be freely available at https://github.com/antonfrancois/Demeter_metamorphosis.
* SEE GSI 2021
Partial Matching in the Space of Varifolds
Mar 23, 2021


Abstract:In computer vision and medical imaging, the problem of matching structures finds numerous applications from automatic annotation to data reconstruction. The data however, while corresponding to the same anatomy, are often very different in topology or shape and might only partially match each other. We introduce a new asymmetric data dissimilarity term for various geometric shapes like sets of curves or surfaces. This term is based on the Varifold shape representation and assesses the embedding of a shape into another one without relying on correspondences between points. It is designed as data attachment for the Large Deformation Diffeomorphic Metric Mapping (LDDMM) framework, allowing to compute meaningful deformation of one shape onto a subset of the other. Registrations are illustrated on sets of synthetic 3D curves, real vascular trees and livers' surfaces from two different modalities: Computed Tomography (CT) and Cone Beam Computed Tomography (CBCT). All experiments show that this data dissimilarity term leads to coherent partial matching despite the topological differences.
Database Annotation with few Examples: An Atlas-based Framework using Diffeomorphic Registration of 3D Trees
Sep 25, 2020



Abstract:Automatic annotation of anatomical structures can help simplify workflow during interventions in numerous clinical applications but usually involves a large amount of annotated data. The complexity of the labeling task, together with the lack of representative data, slows down the development of robust solutions. In this paper, we propose a solution requiring very few annotated cases to label 3D pelvic arterial trees of patients with benign prostatic hyperplasia. We take advantage of Large Deformation Diffeomorphic Metric Mapping (LDDMM) to perform registration based on meaningful deformations from which we build an atlas. Branch pairing is then computed from the atlas to new cases using optimal transport to ensure one-to-one correspondence during the labeling process. To tackle topological variations in the tree, which usually degrades the performance of atlas-based techniques, we propose a simple bottom-up label assignment adapted to the pelvic anatomy. The proposed method achieves 97.6\% labeling precision with only 5 cases for training, while in comparison learning-based methods only reach 82.2\% on such small training sets.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge