Hamed Dashti
AI-Med Group, AI Innovation Center, Sharif University of Technology, Tehran, Iran
CRISPR: Ensemble Model
Mar 05, 2024


Abstract:Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) is a gene editing technology that has revolutionized the fields of biology and medicine. However, one of the challenges of using CRISPR is predicting the on-target efficacy and off-target sensitivity of single-guide RNAs (sgRNAs). This is because most existing methods are trained on separate datasets with different genes and cells, which limits their generalizability. In this paper, we propose a novel ensemble learning method for sgRNA design that is accurate and generalizable. Our method combines the predictions of multiple machine learning models to produce a single, more robust prediction. This approach allows us to learn from a wider range of data, which improves the generalizability of our model. We evaluated our method on a benchmark dataset of sgRNA designs and found that it outperformed existing methods in terms of both accuracy and generalizability. Our results suggest that our method can be used to design sgRNAs with high sensitivity and specificity, even for new genes or cells. This could have important implications for the clinical use of CRISPR, as it would allow researchers to design more effective and safer treatments for a variety of diseases.
Accurate and Rapid Diagnosis of COVID-19 Pneumonia with Batch Effect Removal of Chest CT-Scans and Interpretable Artificial Intelligence
Nov 23, 2020


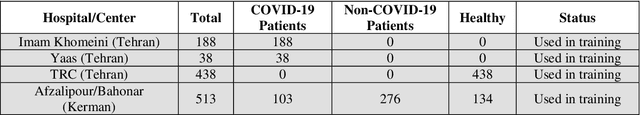
Abstract:Since late 2019, COVID-19 has been spreading over the world and caused the death of many people. The high transmission rate of the virus demands the rapid identification of infected patients to reduce the spread of the disease. The current gold-standard test, Reverse-Transcription Polymerase Chain Reaction (RT-PCR), suffers from a high rate of false negatives. Diagnosis from CT-scan images as an alternative with higher accuracy and sensitivity has the challenge of distinguishing COVID-19 from other lung diseases which demand expert radiologists. In peak times, artificial intelligence (AI) based diagnostic systems can help radiologists to accelerate the process of diagnosis, increase the accuracy, and understand the severity of the disease. We designed an interpretable deep neural network to distinguish healthy people, patients with COVID-19, and patients with other lung diseases from chest CT-scan images. Our model also detects the infected areas of the lung and is able to calculate the percentage of the infected volume. We preprocessed the images to eliminate the batch effect related to CT-scan devices and medical centers and then adopted a weakly supervised method to train the model without having any label for infected parts and any tags for the slices of the CT-scan images that had signs of disease. We trained and evaluated the model on a large dataset of 3359 CT-scan images from 6 medical centers. The model reached a sensitivity of 97.75% and a specificity of 87% in separating healthy people from the diseased and a sensitivity of 98.15% and a specificity of 81.03% in distinguishing COVID-19 from other diseases. The model also reached similar metrics in 1435 samples from 6 unseen medical centers that prove its generalizability. The performance of the model on a large diverse dataset, its generalizability, and interpretability makes it suitable to be used as a diagnostic system.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge