Guang-Uei Hung
FedDA-TSformer: Federated Domain Adaptation with Vision TimeSformer for Left Ventricle Segmentation on Gated Myocardial Perfusion SPECT Image
Feb 23, 2025Abstract:Background and Purpose: Functional assessment of the left ventricle using gated myocardial perfusion (MPS) single-photon emission computed tomography relies on the precise extraction of the left ventricular contours while simultaneously ensuring the security of patient data. Methods: In this paper, we introduce the integration of Federated Domain Adaptation with TimeSformer, named 'FedDA-TSformer' for left ventricle segmentation using MPS. FedDA-TSformer captures spatial and temporal features in gated MPS images, leveraging spatial attention, temporal attention, and federated learning for improved domain adaptation while ensuring patient data security. In detail, we employed Divide-Space-Time-Attention mechanism to extract spatio-temporal correlations from the multi-centered MPS datasets, ensuring that predictions are spatio-temporally consistent. To achieve domain adaptation, we align the model output on MPS from three different centers using local maximum mean discrepancy (LMMD) loss. This approach effectively addresses the dual requirements of federated learning and domain adaptation, enhancing the model's performance during training with multi-site datasets while ensuring the protection of data from different hospitals. Results: Our FedDA-TSformer was trained and evaluated using MPS datasets collected from three hospitals, comprising a total of 150 subjects. Each subject's cardiac cycle was divided into eight gates. The model achieved Dice Similarity Coefficients (DSC) of 0.842 and 0.907 for left ventricular (LV) endocardium and epicardium segmentation, respectively. Conclusion: Our proposed FedDA-TSformer model addresses the challenge of multi-center generalization, ensures patient data privacy protection, and demonstrates effectiveness in left ventricular (LV) segmentation.
Coronary Artery Semantic Labeling using Edge Attention Graph Matching Network
May 21, 2023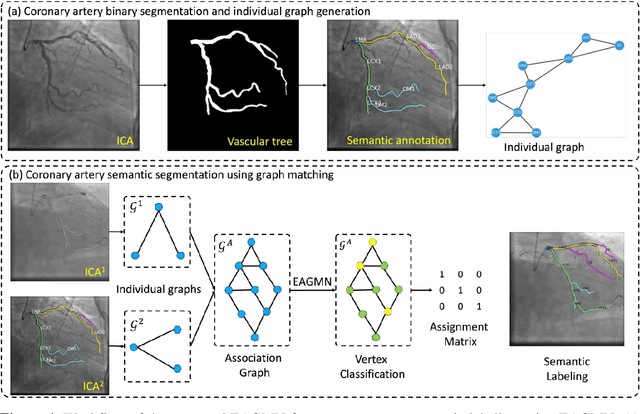
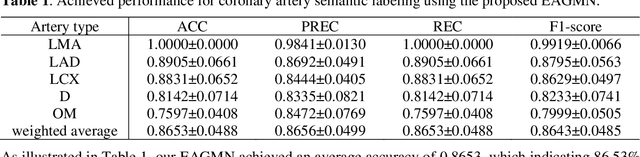
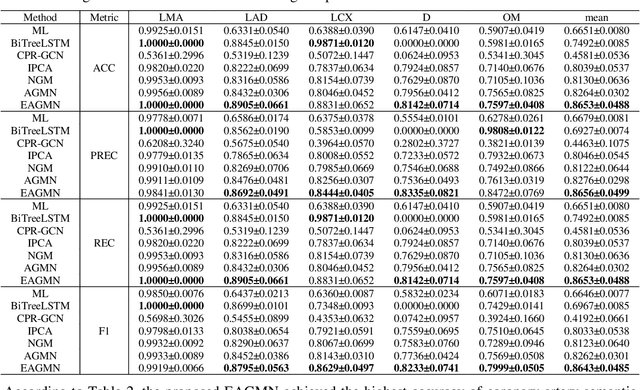
Abstract:Coronary artery disease (CAD) is one of the primary causes leading deaths worldwide. The presence of atherosclerotic lesions in coronary arteries is the underlying pathophysiological basis of CAD, and accurate extraction of individual arterial branches using invasive coronary angiography (ICA) is crucial for stenosis detection and CAD diagnosis. We propose an innovative approach called the Edge Attention Graph Matching Network (EAGMN) for coronary artery semantic labeling. By converting the coronary artery semantic segmentation task into a graph node similarity comparison task, identifying the node-to-node correspondence would assign semantic labels for each arterial branch. More specifically, The EAGMN utilizes the association graph constructed from the two individual graphs as input. Experimental results indicate the EAGMN achieved a weighted accuracy of 0.8653, a weighted precision of 0.8656, a weighted recall of 0.8653 and a weighted F1-score of 0.8643. Furthermore, we employ ZORRO to provide interpretability and explainability of the graph matching for artery semantic labeling. These findings highlight the potential of the EAGMN for accurate and efficient coronary artery semantic labeling using ICAs. By leveraging the inherent characteristics of ICAs and incorporating graph matching techniques, our proposed model provides a promising solution for improving CAD diagnosis and treatment
AGMN: Association Graph-based Graph Matching Network for Coronary Artery Semantic Labeling on Invasive Coronary Angiograms
Jan 11, 2023



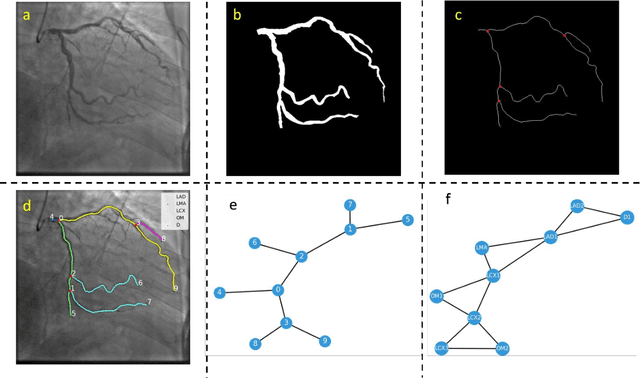
Abstract:Semantic labeling of coronary arterial segments in invasive coronary angiography (ICA) is important for automated assessment and report generation of coronary artery stenosis in the computer-aided diagnosis of coronary artery disease (CAD). Inspired by the training procedure of interventional cardiologists for interpreting the structure of coronary arteries, we propose an association graph-based graph matching network (AGMN) for coronary arterial semantic labeling. We first extract the vascular tree from invasive coronary angiography (ICA) and convert it into multiple individual graphs. Then, an association graph is constructed from two individual graphs where each vertex represents the relationship between two arterial segments. Using the association graph, the AGMN extracts the vertex features by the embedding module, aggregates the features from adjacent vertices and edges by graph convolution network, and decodes the features to generate the semantic mappings between arteries. By learning the mapping of arterial branches between two individual graphs, the unlabeled arterial segments are classified by the labeled segments to achieve semantic labeling. A dataset containing 263 ICAs was employed to train and validate the proposed model, and a five-fold cross-validation scheme was performed. Our AGMN model achieved an average accuracy of 0.8264, an average precision of 0.8276, an average recall of 0.8264, and an average F1-score of 0.8262, which significantly outperformed existing coronary artery semantic labeling methods. In conclusion, we have developed and validated a new algorithm with high accuracy, interpretability, and robustness for coronary artery semantic labeling on ICAs.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge