Giridhar Dasegowda
Scalable Drift Monitoring in Medical Imaging AI
Oct 17, 2024



Abstract:The integration of artificial intelligence (AI) into medical imaging has advanced clinical diagnostics but poses challenges in managing model drift and ensuring long-term reliability. To address these challenges, we develop MMC+, an enhanced framework for scalable drift monitoring, building upon the CheXstray framework that introduced real-time drift detection for medical imaging AI models using multi-modal data concordance. This work extends the original framework's methodologies, providing a more scalable and adaptable solution for real-world healthcare settings and offers a reliable and cost-effective alternative to continuous performance monitoring addressing limitations of both continuous and periodic monitoring methods. MMC+ introduces critical improvements to the original framework, including more robust handling of diverse data streams, improved scalability with the integration of foundation models like MedImageInsight for high-dimensional image embeddings without site-specific training, and the introduction of uncertainty bounds to better capture drift in dynamic clinical environments. Validated with real-world data from Massachusetts General Hospital during the COVID-19 pandemic, MMC+ effectively detects significant data shifts and correlates them with model performance changes. While not directly predicting performance degradation, MMC+ serves as an early warning system, indicating when AI systems may deviate from acceptable performance bounds and enabling timely interventions. By emphasizing the importance of monitoring diverse data streams and evaluating data shifts alongside model performance, this work contributes to the broader adoption and integration of AI solutions in clinical settings.
X-ray Dissectography Improves Lung Nodule Detection
Mar 24, 2022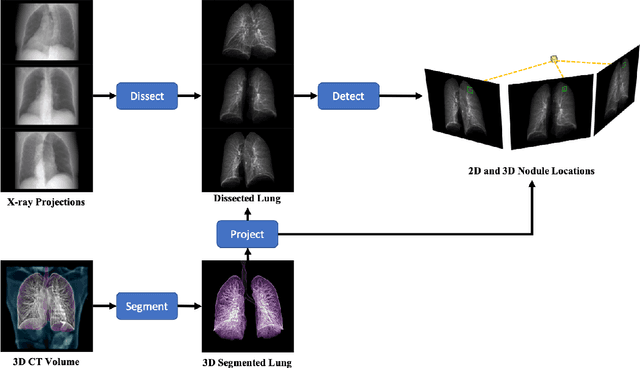
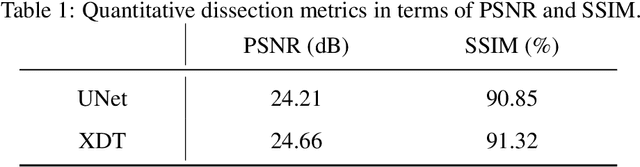
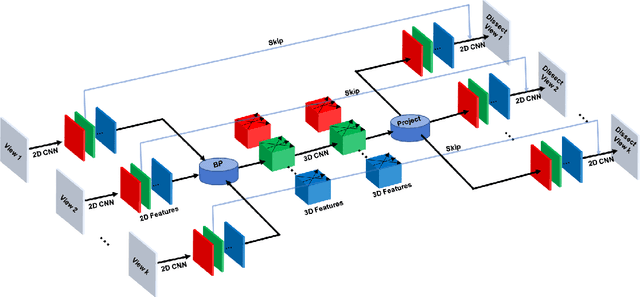
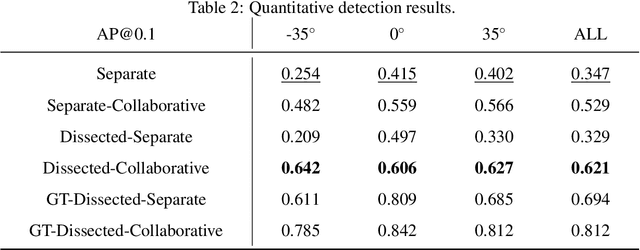
Abstract:Although radiographs are the most frequently used worldwide due to their cost-effectiveness and widespread accessibility, the structural superposition along the x-ray paths often renders suspicious or concerning lung nodules difficult to detect. In this study, we apply "X-ray dissectography" to dissect lungs digitally from a few radiographic projections, suppress the interference of irrelevant structures, and improve lung nodule detectability. For this purpose, a collaborative detection network is designed to localize lung nodules in 2D dissected projections and 3D physical space. Our experimental results show that our approach can significantly improve the average precision by 20+% in comparison with the common baseline that detects lung nodules from original projections using a popular detection network. Potentially, this approach could help re-design the current X-ray imaging protocols and workflows and improve the diagnostic performance of chest radiographs in lung diseases.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge