Gian Gaetano Tartaglia
iASiS: Towards Heterogeneous Big Data Analysis for Personalized Medicine
Jul 09, 2024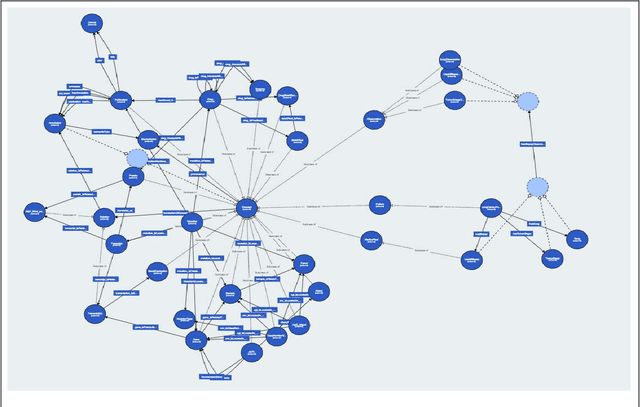
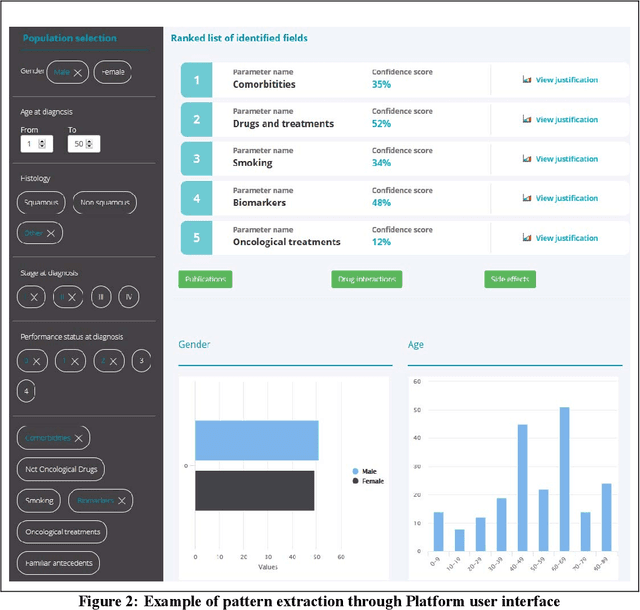
Abstract:The vision of IASIS project is to turn the wave of big biomedical data heading our way into actionable knowledge for decision makers. This is achieved by integrating data from disparate sources, including genomics, electronic health records and bibliography, and applying advanced analytics methods to discover useful patterns. The goal is to turn large amounts of available data into actionable information to authorities for planning public health activities and policies. The integration and analysis of these heterogeneous sources of information will enable the best decisions to be made, allowing for diagnosis and treatment to be personalised to each individual. The project offers a common representation schema for the heterogeneous data sources. The iASiS infrastructure is able to convert clinical notes into usable data, combine them with genomic data, related bibliography, image data and more, and create a global knowledge base. This facilitates the use of intelligent methods in order to discover useful patterns across different resources. Using semantic integration of data gives the opportunity to generate information that is rich, auditable and reliable. This information can be used to provide better care, reduce errors and create more confidence in sharing data, thus providing more insights and opportunities. Data resources for two different disease categories are explored within the iASiS use cases, dementia and lung cancer.
* 6 pages, 2 figures, accepted at 2019 IEEE 32nd International Symposium on Computer-Based Medical Systems (CBMS)
Prediction of gene expression time series and structural analysis of gene regulatory networks using recurrent neural networks
Sep 13, 2021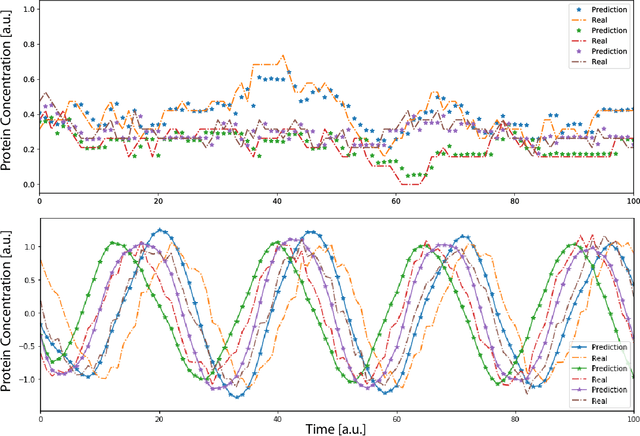
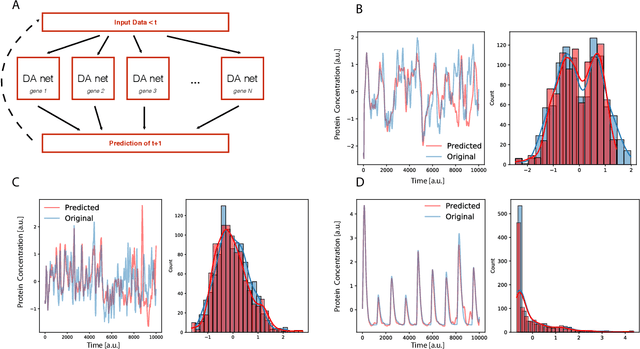
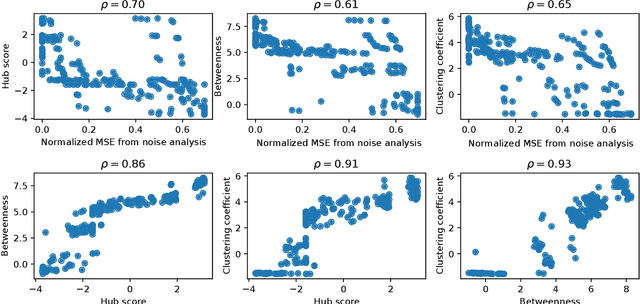
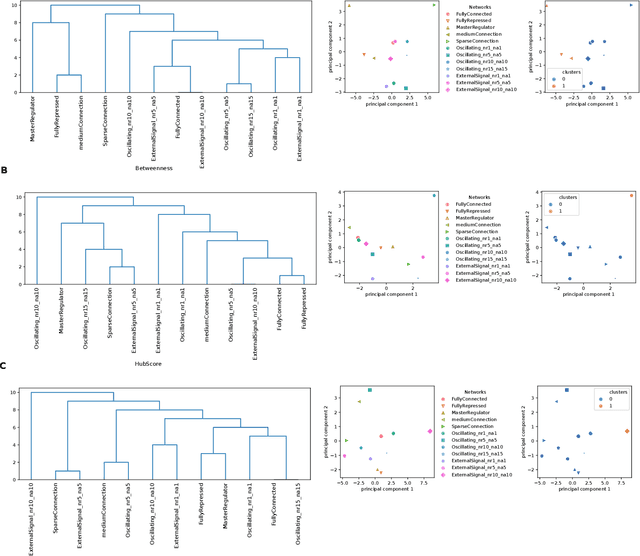
Abstract:Methods for time series prediction and classification of gene regulatory networks (GRNs) from gene expression data have been treated separately so far. The recent emergence of attention-based recurrent neural networks (RNN) models boosted the interpretability of RNN parameters, making them appealing for the understanding of gene interactions. In this work, we generated synthetic time series gene expression data from a range of archetypal GRNs and we relied on a dual attention RNN to predict the gene temporal dynamics. We show that the prediction is extremely accurate for GRNs with different architectures. Next, we focused on the attention mechanism of the RNN and, using tools from graph theory, we found that its graph properties allow to hierarchically distinguish different architectures of the GRN. We show that the GRNs respond differently to the addition of noise in the prediction by the RNN and we relate the noise response to the analysis of the attention mechanism. In conclusion, this work provides a a way to understand and exploit the attention mechanism of RNN and it paves the way to RNN-based methods for time series prediction and inference of GRNs from gene expression data.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge