Daniele Ravi
for the Alzheimer's Disease Neuroimaging Initiative
An efficient semi-supervised quality control system trained using physics-based MRI-artefact generators and adversarial training
Jun 07, 2022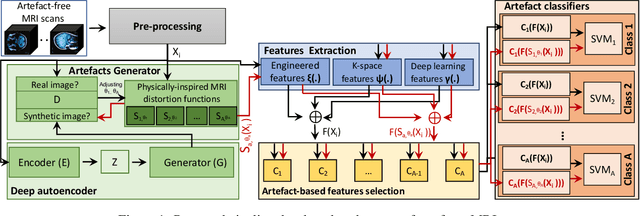
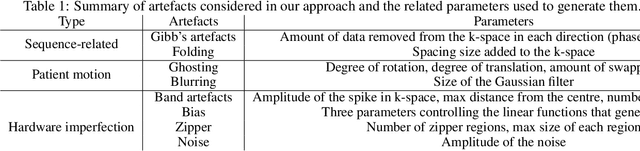
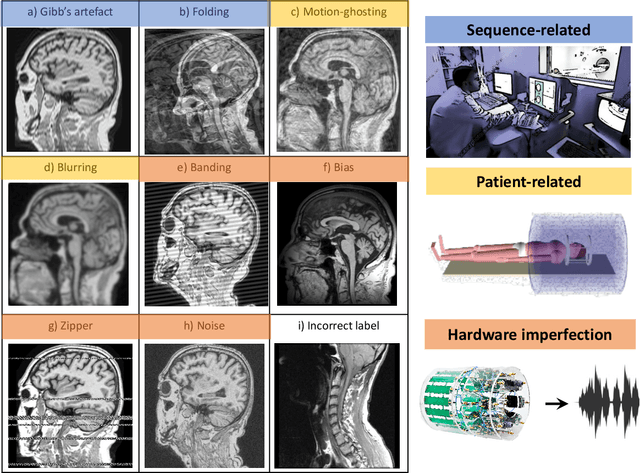
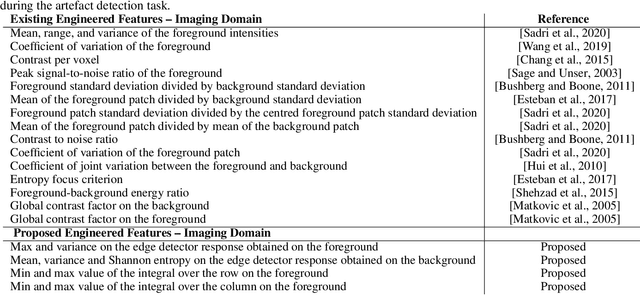
Abstract:Large medical imaging data sets are becoming increasingly available. A common challenge in these data sets is to ensure that each sample meets minimum quality requirements devoid of significant artefacts. Despite a wide range of existing automatic methods having been developed to identify imperfections and artefacts in medical imaging, they mostly rely on data-hungry methods. In particular, the lack of sufficient scans with artefacts available for training has created a barrier in designing and deploying machine learning in clinical research. To tackle this problem, we propose a novel framework having four main components: (1) a set of artefact generators inspired by magnetic resonance physics to corrupt brain MRI scans and augment a training dataset, (2) a set of abstract and engineered features to represent images compactly, (3) a feature selection process that depends on the class of artefact to improve classification performance, and (4) a set of Support Vector Machine (SVM) classifiers trained to identify artefacts. Our novel contributions are threefold: first, we use the novel physics-based artefact generators to generate synthetic brain MRI scans with controlled artefacts as a data augmentation technique. This will avoid the labour-intensive collection and labelling process of scans with rare artefacts. Second, we propose a large pool of abstract and engineered image features developed to identify 9 different artefacts for structural MRI. Finally, we use an artefact-based feature selection block that, for each class of artefacts, finds the set of features that provide the best classification performance. We performed validation experiments on a large data set of scans with artificially-generated artefacts, and in a multiple sclerosis clinical trial where real artefacts were identified by experts, showing that the proposed pipeline outperforms traditional methods.
Degenerative Adversarial NeuroImage Nets for 3D Simulations: Application in Longitudinal MRI
Dec 03, 2019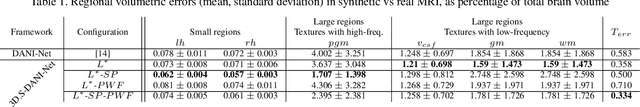
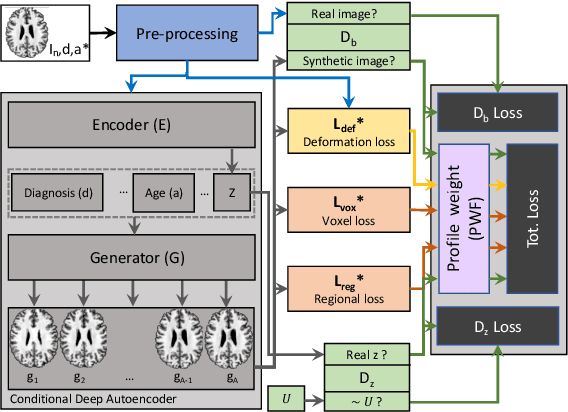
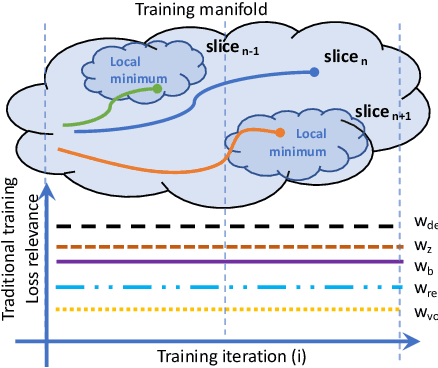
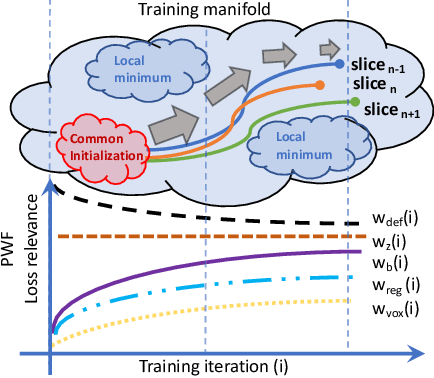
Abstract:The recent success of deep learning together with the availability of large medical imaging datasets have enabled researchers to improve our understanding of complex chronic medical conditions such as neurodegenerative diseases. The possibility of predicting realistic and accurate images would be a breakthrough for many clinical healthcare applications. However, current image simulators designed to model neurodegenerative disease progression present limitations that preclude their utility in clinical practice. These limitations include personalization of disease progression and the ability to synthesize spatiotemporal images in high resolution. In particular, memory limitations prohibit full 3D image models, necessitating various techniques to discard spatiotemporal information, such as patch-based approaches. In this work, we introduce a novel technique to address this challenge, called Profile Weight Functions (PWF). We demonstrate its effectiveness integrated within our new deep learning framework, showing that it enables the extension to 3D of a recent state-of-the-art 2D approach. To our knowledge, we are the first to implement a personalized disease progression simulator able to predict accurate, personalised, high-resolution, 3D MRI. In particular, we trained a model of ageing and Alzheimer's disease progression using 9652 T1-weighted (longitudinal) MRI from the Alzheimer's Disease Neuroimaging Initiative (ADNI) dataset and validated on a separate test set of 1283 MRI (also from ADNI, random partition). We validated our model by analyzing its capability to synthesize MRI that produce accurate volumes of specific brain regions associated with neurodegeneration. Our experiments demonstrate the effectiveness of our solution to provide a 3D simulation that produces accurate and convincing synthetic MRI that emulate ageing and disease progression.
Degenerative Adversarial NeuroImage Nets: Generating Images that Mimic Disease Progression
Jul 05, 2019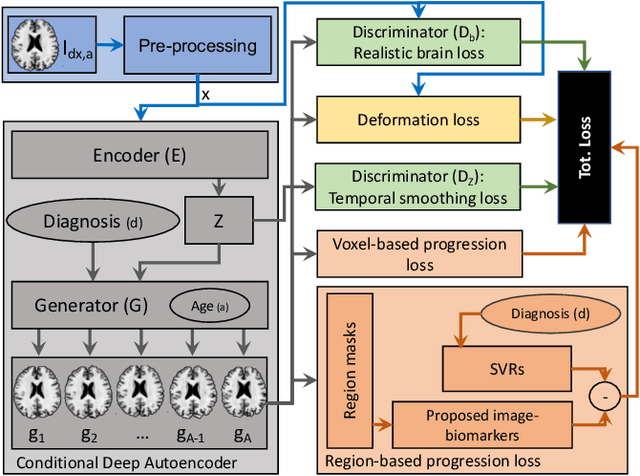
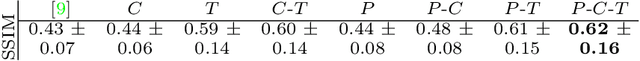
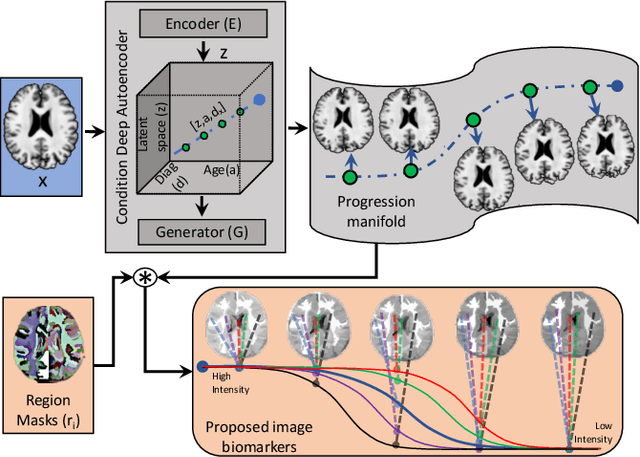
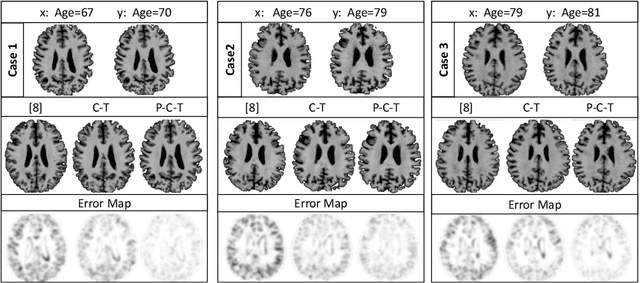
Abstract:Simulating images representative of neurodegenerative diseases is important for predicting patient outcomes and for validation of computational models of disease progression. This capability is valuable for secondary prevention clinical trials where outcomes and screening criteria involve neuroimaging. Traditional computational methods are limited by imposing a parametric model for atrophy and are extremely resource-demanding. Recent advances in deep learning have yielded data-driven models for longitudinal studies (e.g., face ageing) that are capable of generating synthetic images in real-time. Similar solutions can be used to model trajectories of atrophy in the brain, although new challenges need to be addressed to ensure accurate disease progression modelling. Here we propose Degenerative Adversarial NeuroImage Net (DaniNet) --- a new deep learning approach that learns to emulate the effect of neurodegeneration on MRI. DaniNet uses an underlying set of Support Vector Regressors (SVRs) trained to capture the patterns of regional intensity changes that accompany disease progression. DaniNet produces whole output images, consisting of 2D-MRI slices that are constrained to match regional predictions from the SVRs. DaniNet is also able to condition the progression on non-imaging characteristics (age, diagnosis, etc.) while it maintains the unique brain morphology of individuals. Adversarial training ensures realistic brain images and smooth temporal progression. We train our model using 9652 T1-weighted (longitudinal) MRI extracted from the Alzheimer's Disease Neuroimaging Initiative (ADNI) dataset. We perform quantitative and qualitative evaluations on a separate test set of 1283 images (also from ADNI) demonstrating the ability of DaniNet to produce accurate and convincing synthetic images that emulate disease progression.
Artificial Intelligence and Robotics
Mar 28, 2018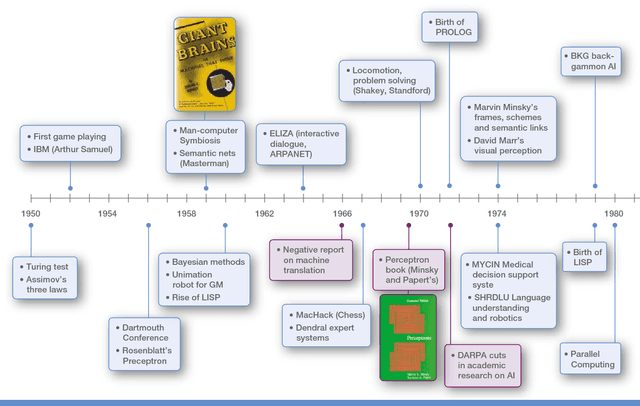
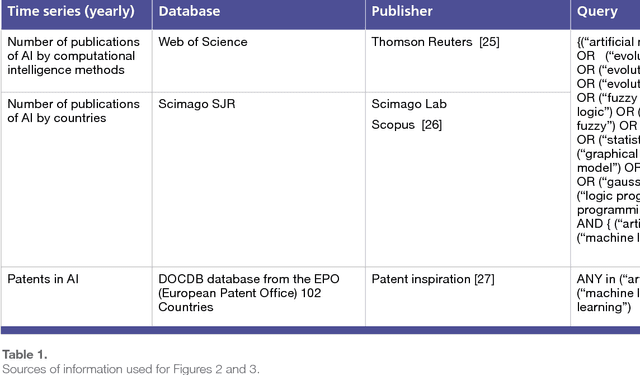
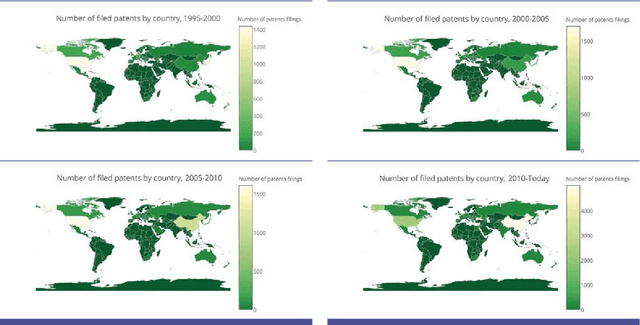
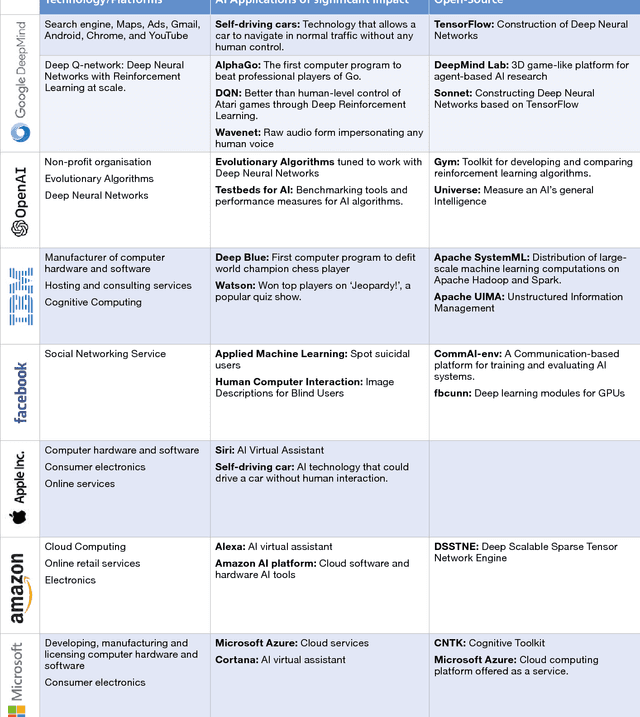
Abstract:The recent successes of AI have captured the wildest imagination of both the scientific communities and the general public. Robotics and AI amplify human potentials, increase productivity and are moving from simple reasoning towards human-like cognitive abilities. Current AI technologies are used in a set area of applications, ranging from healthcare, manufacturing, transport, energy, to financial services, banking, advertising, management consulting and government agencies. The global AI market is around 260 billion USD in 2016 and it is estimated to exceed 3 trillion by 2024. To understand the impact of AI, it is important to draw lessons from it's past successes and failures and this white paper provides a comprehensive explanation of the evolution of AI, its current status and future directions.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge