Christiaan Viviers
Zero-Shot Image Anomaly Detection Using Generative Foundation Models
Jul 30, 2025Abstract:Detecting out-of-distribution (OOD) inputs is pivotal for deploying safe vision systems in open-world environments. We revisit diffusion models, not as generators, but as universal perceptual templates for OOD detection. This research explores the use of score-based generative models as foundational tools for semantic anomaly detection across unseen datasets. Specifically, we leverage the denoising trajectories of Denoising Diffusion Models (DDMs) as a rich source of texture and semantic information. By analyzing Stein score errors, amplified through the Structural Similarity Index Metric (SSIM), we introduce a novel method for identifying anomalous samples without requiring re-training on each target dataset. Our approach improves over state-of-the-art and relies on training a single model on one dataset -- CelebA -- which we find to be an effective base distribution, even outperforming more commonly used datasets like ImageNet in several settings. Experimental results show near-perfect performance on some benchmarks, with notable headroom on others, highlighting both the strength and future potential of generative foundation models in anomaly detection.
Symmetrical Flow Matching: Unified Image Generation, Segmentation, and Classification with Score-Based Generative Models
Jun 12, 2025Abstract:Flow Matching has emerged as a powerful framework for learning continuous transformations between distributions, enabling high-fidelity generative modeling. This work introduces Symmetrical Flow Matching (SymmFlow), a new formulation that unifies semantic segmentation, classification, and image generation within a single model. Using a symmetric learning objective, SymmFlow models forward and reverse transformations jointly, ensuring bi-directional consistency, while preserving sufficient entropy for generative diversity. A new training objective is introduced to explicitly retain semantic information across flows, featuring efficient sampling while preserving semantic structure, allowing for one-step segmentation and classification without iterative refinement. Unlike previous approaches that impose strict one-to-one mapping between masks and images, SymmFlow generalizes to flexible conditioning, supporting both pixel-level and image-level class labels. Experimental results on various benchmarks demonstrate that SymmFlow achieves state-of-the-art performance on semantic image synthesis, obtaining FID scores of 11.9 on CelebAMask-HQ and 7.0 on COCO-Stuff with only 25 inference steps. Additionally, it delivers competitive results on semantic segmentation and shows promising capabilities in classification tasks. The code will be publicly available.
AdverX-Ray: Ensuring X-Ray Integrity Through Frequency-Sensitive Adversarial VAEs
Feb 23, 2025Abstract:Ensuring the quality and integrity of medical images is crucial for maintaining diagnostic accuracy in deep learning-based Computer-Aided Diagnosis and Computer-Aided Detection (CAD) systems. Covariate shifts are subtle variations in the data distribution caused by different imaging devices or settings and can severely degrade model performance, similar to the effects of adversarial attacks. Therefore, it is vital to have a lightweight and fast method to assess the quality of these images prior to using CAD models. AdverX-Ray addresses this need by serving as an image-quality assessment layer, designed to detect covariate shifts effectively. This Adversarial Variational Autoencoder prioritizes the discriminator's role, using the suboptimal outputs of the generator as negative samples to fine-tune the discriminator's ability to identify high-frequency artifacts. Images generated by adversarial networks often exhibit severe high-frequency artifacts, guiding the discriminator to focus excessively on these components. This makes the discriminator ideal for this approach. Trained on patches from X-ray images of specific machine models, AdverX-Ray can evaluate whether a scan matches the training distribution, or if a scan from the same machine is captured under different settings. Extensive comparisons with various OOD detection methods show that AdverX-Ray significantly outperforms existing techniques, achieving a 96.2% average AUROC using only 64 random patches from an X-ray. Its lightweight and fast architecture makes it suitable for real-time applications, enhancing the reliability of medical imaging systems. The code and pretrained models are publicly available.
DisCoPatch: Batch Statistics Are All You Need For OOD Detection, But Only If You Can Trust Them
Jan 14, 2025Abstract:Out-of-distribution (OOD) detection holds significant importance across many applications. While semantic and domain-shift OOD problems are well-studied, this work focuses on covariate shifts - subtle variations in the data distribution that can degrade machine learning performance. We hypothesize that detecting these subtle shifts can improve our understanding of in-distribution boundaries, ultimately improving OOD detection. In adversarial discriminators trained with Batch Normalization (BN), real and adversarial samples form distinct domains with unique batch statistics - a property we exploit for OOD detection. We introduce DisCoPatch, an unsupervised Adversarial Variational Autoencoder (VAE) framework that harnesses this mechanism. During inference, batches consist of patches from the same image, ensuring a consistent data distribution that allows the model to rely on batch statistics. DisCoPatch uses the VAE's suboptimal outputs (generated and reconstructed) as negative samples to train the discriminator, thereby improving its ability to delineate the boundary between in-distribution samples and covariate shifts. By tightening this boundary, DisCoPatch achieves state-of-the-art results in public OOD detection benchmarks. The proposed model not only excels in detecting covariate shifts, achieving 95.5% AUROC on ImageNet-1K(-C) but also outperforms all prior methods on public Near-OOD (95.0%) benchmarks. With a compact model size of 25MB, it achieves high OOD detection performance at notably lower latency than existing methods, making it an efficient and practical solution for real-world OOD detection applications. The code will be made publicly available
Can Your Generative Model Detect Out-of-Distribution Covariate Shift?
Sep 04, 2024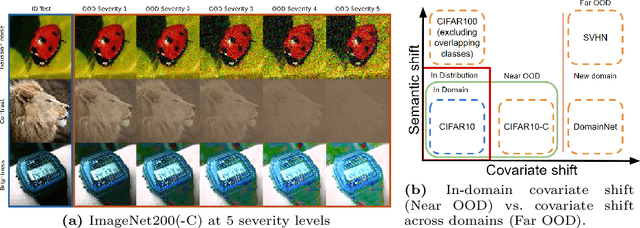



Abstract:Detecting Out-of-Distribution~(OOD) sensory data and covariate distribution shift aims to identify new test examples with different high-level image statistics to the captured, normal and In-Distribution (ID) set. Existing OOD detection literature largely focuses on semantic shift with little-to-no consensus over covariate shift. Generative models capture the ID data in an unsupervised manner, enabling them to effectively identify samples that deviate significantly from this learned distribution, irrespective of the downstream task. In this work, we elucidate the ability of generative models to detect and quantify domain-specific covariate shift through extensive analyses that involves a variety of models. To this end, we conjecture that it is sufficient to detect most occurring sensory faults (anomalies and deviations in global signals statistics) by solely modeling high-frequency signal-dependent and independent details. We propose a novel method, CovariateFlow, for OOD detection, specifically tailored to covariate heteroscedastic high-frequency image-components using conditional Normalizing Flows (cNFs). Our results on CIFAR10 vs. CIFAR10-C and ImageNet200 vs. ImageNet200-C demonstrate the effectiveness of the method by accurately detecting OOD covariate shift. This work contributes to enhancing the fidelity of imaging systems and aiding machine learning models in OOD detection in the presence of covariate shift.
Segmentation-based Assessment of Tumor-Vessel Involvement for Surgical Resectability Prediction of Pancreatic Ductal Adenocarcinoma
Oct 01, 2023



Abstract:Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive cancer with limited treatment options. This research proposes a workflow and deep learning-based segmentation models to automatically assess tumor-vessel involvement, a key factor in determining tumor resectability. Correct assessment of resectability is vital to determine treatment options. The proposed workflow involves processing CT scans to segment the tumor and vascular structures, analyzing spatial relationships and the extent of vascular involvement, which follows a similar way of working as expert radiologists in PDAC assessment. Three segmentation architectures (nnU-Net, 3D U-Net, and Probabilistic 3D U-Net) achieve a high accuracy in segmenting veins, arteries, and the tumor. The segmentations enable automated detection of tumor involvement with high accuracy (0.88 sensitivity and 0.86 specificity) and automated computation of the degree of tumor-vessel contact. Additionally, due to significant inter-observer variability in these important structures, we present the uncertainty captured by each of the models to further increase insights into the predicted involvement. This result provides clinicians with a clear indication of tumor-vessel involvement and may be used to facilitate more informed decision-making for surgical interventions. The proposed method offers a valuable tool for improving patient outcomes, personalized treatment strategies and survival rates in pancreatic cancer.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge