Christiaan G. A. Viviers
Advancing 6-DoF Instrument Pose Estimation in Variable X-Ray Imaging Geometries
May 19, 2024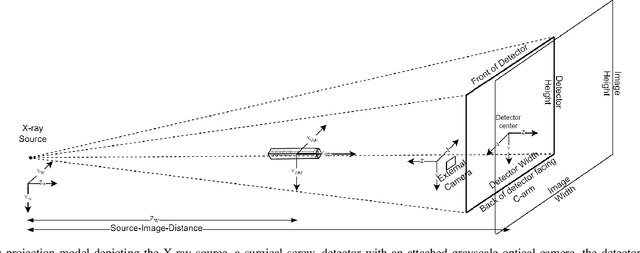
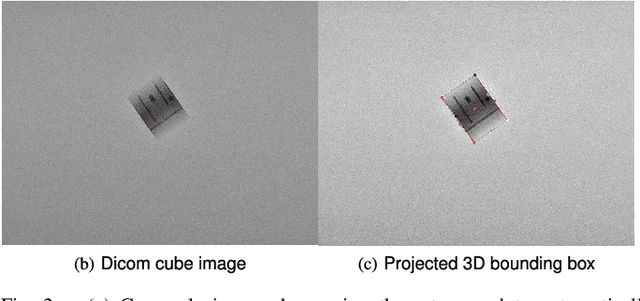
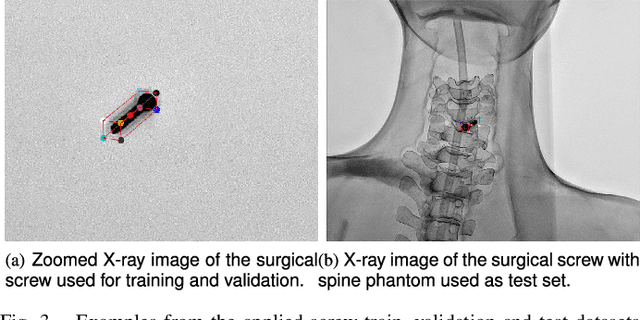
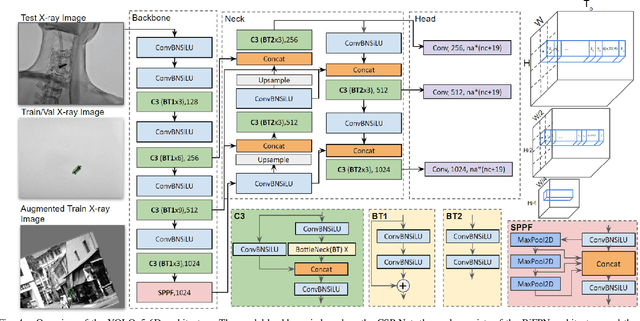
Abstract:Accurate 6-DoF pose estimation of surgical instruments during minimally invasive surgeries can substantially improve treatment strategies and eventual surgical outcome. Existing deep learning methods have achieved accurate results, but they require custom approaches for each object and laborious setup and training environments often stretching to extensive simulations, whilst lacking real-time computation. We propose a general-purpose approach of data acquisition for 6-DoF pose estimation tasks in X-ray systems, a novel and general purpose YOLOv5-6D pose architecture for accurate and fast object pose estimation and a complete method for surgical screw pose estimation under acquisition geometry consideration from a monocular cone-beam X-ray image. The proposed YOLOv5-6D pose model achieves competitive results on public benchmarks whilst being considerably faster at 42 FPS on GPU. In addition, the method generalizes across varying X-ray acquisition geometry and semantic image complexity to enable accurate pose estimation over different domains. Finally, the proposed approach is tested for bone-screw pose estimation for computer-aided guidance during spine surgeries. The model achieves a 92.41% by the 0.1 ADD-S metric, demonstrating a promising approach for enhancing surgical precision and patient outcomes. The code for YOLOv5-6D is publicly available at https://github.com/cviviers/YOLOv5-6D-Pose
* Early author version of paper. Refer to the full paper at https://ieeexplore.ieee.org/document/10478293
Investigating and Improving Latent Density Segmentation Models for Aleatoric Uncertainty Quantification in Medical Imaging
Aug 15, 2023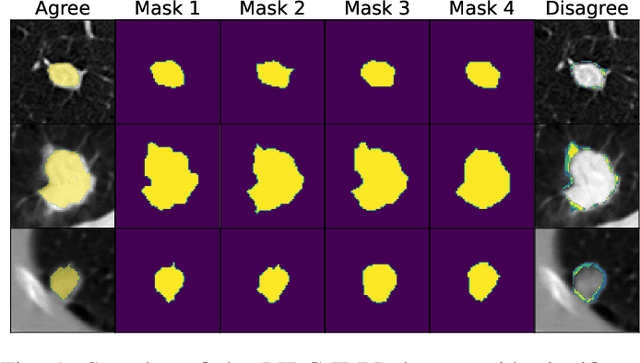


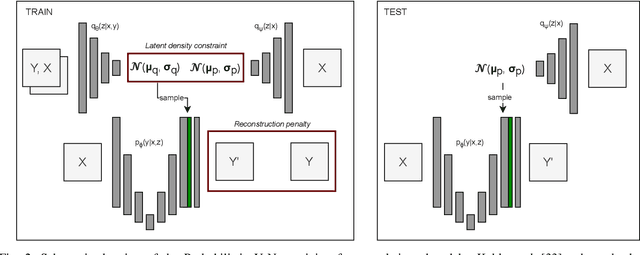
Abstract:Data uncertainties, such as sensor noise or occlusions, can introduce irreducible ambiguities in images, which result in varying, yet plausible, semantic hypotheses. In Machine Learning, this ambiguity is commonly referred to as aleatoric uncertainty. Latent density models can be utilized to address this problem in image segmentation. The most popular approach is the Probabilistic U-Net (PU-Net), which uses latent Normal densities to optimize the conditional data log-likelihood Evidence Lower Bound. In this work, we demonstrate that the PU- Net latent space is severely inhomogenous. As a result, the effectiveness of gradient descent is inhibited and the model becomes extremely sensitive to the localization of the latent space samples, resulting in defective predictions. To address this, we present the Sinkhorn PU-Net (SPU-Net), which uses the Sinkhorn Divergence to promote homogeneity across all latent dimensions, effectively improving gradient-descent updates and model robustness. Our results show that by applying this on public datasets of various clinical segmentation problems, the SPU-Net receives up to 11% performance gains compared against preceding latent variable models for probabilistic segmentation on the Hungarian-Matched metric. The results indicate that by encouraging a homogeneous latent space, one can significantly improve latent density modeling for medical image segmentation.
Probabilistic 3D segmentation for aleatoric uncertainty quantification in full 3D medical data
May 01, 2023Abstract:Uncertainty quantification in medical images has become an essential addition to segmentation models for practical application in the real world. Although there are valuable developments in accurate uncertainty quantification methods using 2D images and slices of 3D volumes, in clinical practice, the complete 3D volumes (such as CT and MRI scans) are used to evaluate and plan the medical procedure. As a result, the existing 2D methods miss the rich 3D spatial information when resolving the uncertainty. A popular approach for quantifying the ambiguity in the data is to learn a distribution over the possible hypotheses. In recent work, this ambiguity has been modeled to be strictly Gaussian. Normalizing Flows (NFs) are capable of modelling more complex distributions and thus, better fit the embedding space of the data. To this end, we have developed a 3D probabilistic segmentation framework augmented with NFs, to enable capturing the distributions of various complexity. To test the proposed approach, we evaluate the model on the LIDC-IDRI dataset for lung nodule segmentation and quantify the aleatoric uncertainty introduced by the multi-annotator setting and inherent ambiguity in the CT data. Following this approach, we are the first to present a 3D Squared Generalized Energy Distance (GED) of 0.401 and a high 0.468 Hungarian-matched 3D IoU. The obtained results reveal the value in capturing the 3D uncertainty, using a flexible posterior distribution augmented with a Normalizing Flow. Finally, we present the aleatoric uncertainty in a visual manner with the aim to provide clinicians with additional insight into data ambiguity and facilitating more informed decision-making.
Towards real-time 6D pose estimation of objects in single-view cone-beam X-ray
Nov 06, 2022Abstract:Deep learning-based pose estimation algorithms can successfully estimate the pose of objects in an image, especially in the field of color images. 6D Object pose estimation based on deep learning models for X-ray images often use custom architectures that employ extensive CAD models and simulated data for training purposes. Recent RGB-based methods opt to solve pose estimation problems using small datasets, making them more attractive for the X-ray domain where medical data is scarcely available. We refine an existing RGB-based model (SingleShotPose) to estimate the 6D pose of a marked cube from grayscale X-ray images by creating a generic solution trained on only real X-ray data and adjusted for X-ray acquisition geometry. The model regresses 2D control points and calculates the pose through 2D/3D correspondences using Perspective-n-Point(PnP), allowing a single trained model to be used across all supporting cone-beam-based X-ray geometries. Since modern X-ray systems continuously adjust acquisition parameters during a procedure, it is essential for such a pose estimation network to consider these parameters in order to be deployed successfully and find a real use case. With a 5-cm/5-degree accuracy of 93% and an average 3D rotation error of 2.2 degrees, the results of the proposed approach are comparable with state-of-the-art alternatives, while requiring significantly less real training examples and being applicable in real-time applications.
Efficient Out-of-Distribution Detection of Melanoma with Wavelet-based Normalizing Flows
Aug 10, 2022



Abstract:Melanoma is a serious form of skin cancer with high mortality rate at later stages. Fortunately, when detected early, the prognosis of melanoma is promising and malignant melanoma incidence rates are relatively low. As a result, datasets are heavily imbalanced which complicates training current state-of-the-art supervised classification AI models. We propose to use generative models to learn the benign data distribution and detect Out-of-Distribution (OOD) malignant images through density estimation. Normalizing Flows (NFs) are ideal candidates for OOD detection due to their ability to compute exact likelihoods. Nevertheless, their inductive biases towards apparent graphical features rather than semantic context hamper accurate OOD detection. In this work, we aim at using these biases with domain-level knowledge of melanoma, to improve likelihood-based OOD detection of malignant images. Our encouraging results demonstrate potential for OOD detection of melanoma using NFs. We achieve a 9% increase in Area Under Curve of the Receiver Operating Characteristics by using wavelet-based NFs. This model requires significantly less parameters for inference making it more applicable on edge devices. The proposed methodology can aid medical experts with diagnosis of skin-cancer patients and continuously increase survival rates. Furthermore, this research paves the way for other areas in oncology with similar data imbalance issues.
Improved Pancreatic Tumor Detection by Utilizing Clinically-Relevant Secondary Features
Aug 06, 2022

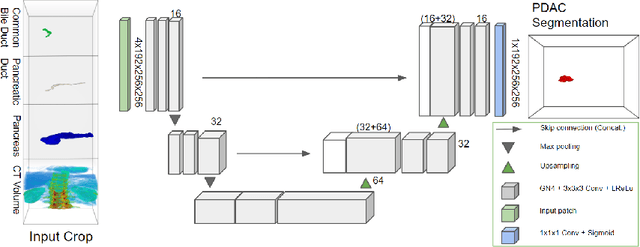

Abstract:Pancreatic cancer is one of the global leading causes of cancer-related deaths. Despite the success of Deep Learning in computer-aided diagnosis and detection (CAD) methods, little attention has been paid to the detection of Pancreatic Cancer. We propose a method for detecting pancreatic tumor that utilizes clinically-relevant features in the surrounding anatomical structures, thereby better aiming to exploit the radiologist's knowledge compared to other, conventional deep learning approaches. To this end, we collect a new dataset consisting of 99 cases with pancreatic ductal adenocarcinoma (PDAC) and 97 control cases without any pancreatic tumor. Due to the growth pattern of pancreatic cancer, the tumor may not be always visible as a hypodense lesion, therefore experts refer to the visibility of secondary external features that may indicate the presence of the tumor. We propose a method based on a U-Net-like Deep CNN that exploits the following external secondary features: the pancreatic duct, common bile duct and the pancreas, along with a processed CT scan. Using these features, the model segments the pancreatic tumor if it is present. This segmentation for classification and localization approach achieves a performance of 99% sensitivity (one case missed) and 99% specificity, which realizes a 5% increase in sensitivity over the previous state-of-the-art method. The model additionally provides location information with reasonable accuracy and a shorter inference time compared to previous PDAC detection methods. These results offer a significant performance improvement and highlight the importance of incorporating the knowledge of the clinical expert when developing novel CAD methods.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge