Cheng Boon
Deep learning in magnetic resonance prostate segmentation: A review and a new perspective
Nov 16, 2020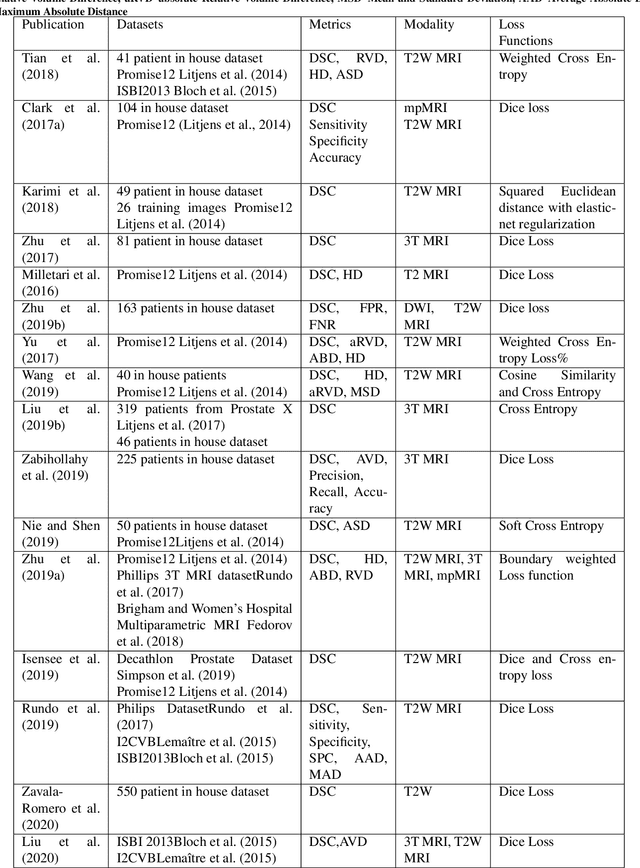
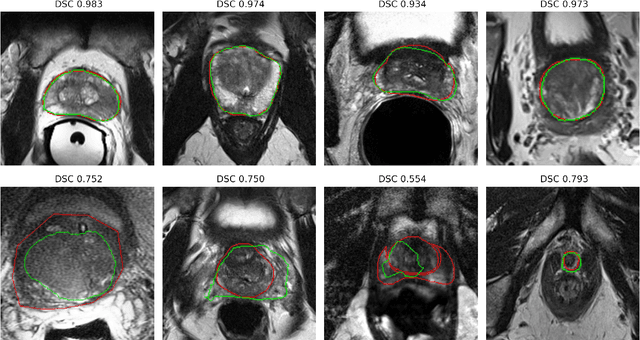
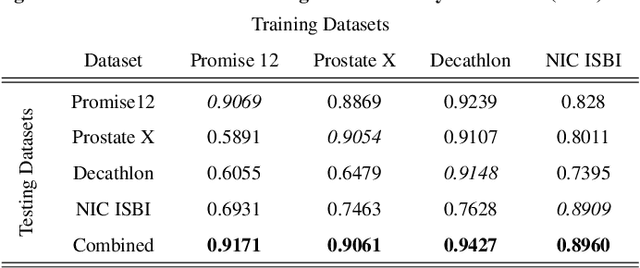
Abstract:Prostate radiotherapy is a well established curative oncology modality, which in future will use Magnetic Resonance Imaging (MRI)-based radiotherapy for daily adaptive radiotherapy target definition. However the time needed to delineate the prostate from MRI data accurately is a time consuming process. Deep learning has been identified as a potential new technology for the delivery of precision radiotherapy in prostate cancer, where accurate prostate segmentation helps in cancer detection and therapy. However, the trained models can be limited in their application to clinical setting due to different acquisition protocols, limited publicly available datasets, where the size of the datasets are relatively small. Therefore, to explore the field of prostate segmentation and to discover a generalisable solution, we review the state-of-the-art deep learning algorithms in MR prostate segmentation; provide insights to the field by discussing their limitations and strengths; and propose an optimised 2D U-Net for MR prostate segmentation. We evaluate the performance on four publicly available datasets using Dice Similarity Coefficient (DSC) as performance metric. Our experiments include within dataset evaluation and cross-dataset evaluation. The best result is achieved by composite evaluation (DSC of 0.9427 on Decathlon test set) and the poorest result is achieved by cross-dataset evaluation (DSC of 0.5892, Prostate X training set, Promise 12 testing set). We outline the challenges and provide recommendations for future work. Our research provides a new perspective to MR prostate segmentation and more importantly, we provide standardised experiment settings for researchers to evaluate their algorithms. Our code is available at https://github.com/AIEMMU/MRI\_Prostate.
Deep learning to achieve clinically applicable segmentation of head and neck anatomy for radiotherapy
Sep 12, 2018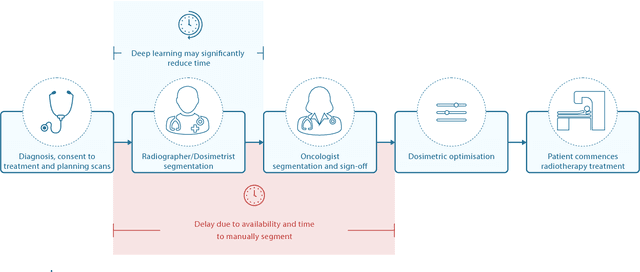
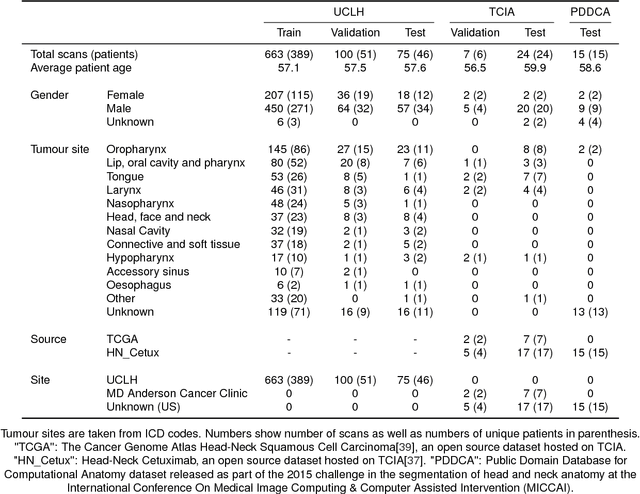
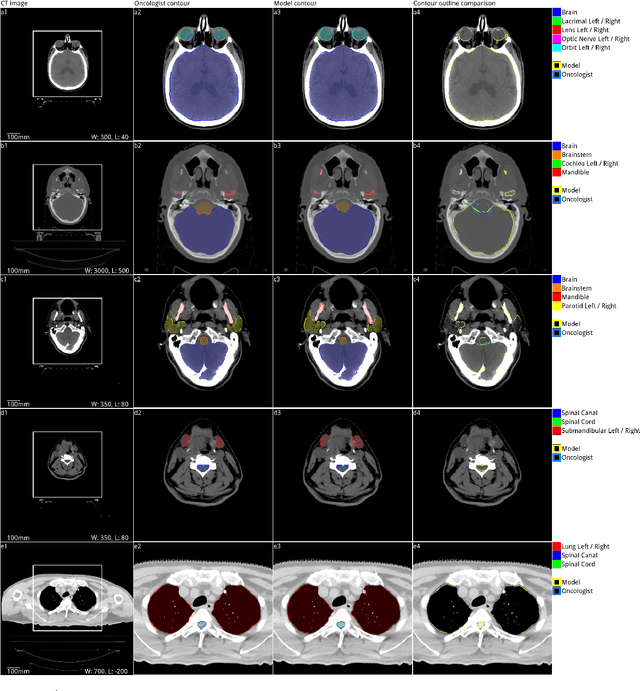
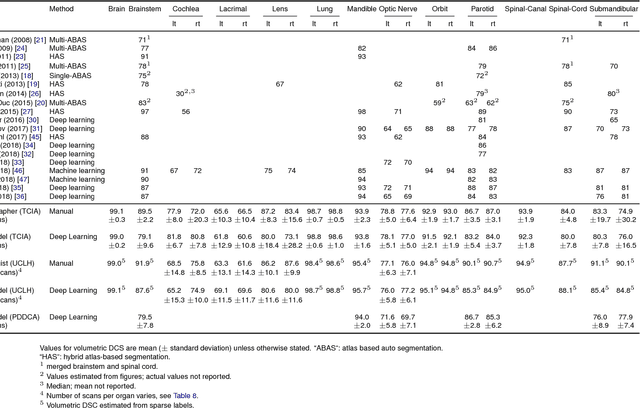
Abstract:Over half a million individuals are diagnosed with head and neck cancer each year worldwide. Radiotherapy is an important curative treatment for this disease, but it requires manually intensive delineation of radiosensitive organs at risk (OARs). This planning process can delay treatment commencement. While auto-segmentation algorithms offer a potentially time-saving solution, the challenges in defining, quantifying and achieving expert performance remain. Adopting a deep learning approach, we demonstrate a 3D U-Net architecture that achieves performance similar to experts in delineating a wide range of head and neck OARs. The model was trained on a dataset of 663 deidentified computed tomography (CT) scans acquired in routine clinical practice and segmented according to consensus OAR definitions. We demonstrate its generalisability through application to an independent test set of 24 CT scans available from The Cancer Imaging Archive collected at multiple international sites previously unseen to the model, each segmented by two independent experts and consisting of 21 OARs commonly segmented in clinical practice. With appropriate validation studies and regulatory approvals, this system could improve the effectiveness of radiotherapy pathways.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge