Cecilia G. Morales
Multimodal Bayesian Network for Robust Assessment of Casualties in Autonomous Triage
Dec 21, 2025Abstract:Mass Casualty Incidents can overwhelm emergency medical systems and resulting delays or errors in the assessment of casualties can lead to preventable deaths. We present a decision support framework that fuses outputs from multiple computer vision models, estimating signs of severe hemorrhage, respiratory distress, physical alertness, or visible trauma, into a Bayesian network constructed entirely from expert-defined rules. Unlike traditional data-driven models, our approach does not require training data, supports inference with incomplete information, and is robust to noisy or uncertain observations. We report performance for two missions involving 11 and 9 casualties, respectively, where our Bayesian network model substantially outperformed vision-only baselines during evaluation of our system in the DARPA Triage Challenge (DTC) field scenarios. The accuracy of physiological assessment improved from 15% to 42% in the first scenario and from 19% to 46% in the second, representing nearly threefold increase in performance. More importantly, overall triage accuracy increased from 14% to 53% in all patients, while the diagnostic coverage of the system expanded from 31% to 95% of the cases requiring assessment. These results demonstrate that expert-knowledge-guided probabilistic reasoning can significantly enhance automated triage systems, offering a promising approach to supporting emergency responders in MCIs. This approach enabled Team Chiron to achieve 4th place out of 11 teams during the 1st physical round of the DTC.
Automatic Cannulation of Femoral Vessels in a Porcine Shock Model
Jun 17, 2025Abstract:Rapid and reliable vascular access is critical in trauma and critical care. Central vascular catheterization enables high-volume resuscitation, hemodynamic monitoring, and advanced interventions like ECMO and REBOA. While peripheral access is common, central access is often necessary but requires specialized ultrasound-guided skills, posing challenges in prehospital settings. The complexity arises from deep target vessels and the precision needed for needle placement. Traditional techniques, like the Seldinger method, demand expertise to avoid complications. Despite its importance, ultrasound-guided central access is underutilized due to limited field expertise. While autonomous needle insertion has been explored for peripheral vessels, only semi-autonomous methods exist for femoral access. This work advances toward full automation, integrating robotic ultrasound for minimally invasive emergency procedures. Our key contribution is the successful femoral vein and artery cannulation in a porcine hemorrhagic shock model.
* 2 pages, 2 figures, conference
Bifurcation Identification for Ultrasound-driven Robotic Cannulation
Sep 10, 2024
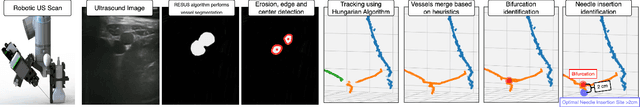


Abstract:In trauma and critical care settings, rapid and precise intravascular access is key to patients' survival. Our research aims at ensuring this access, even when skilled medical personnel are not readily available. Vessel bifurcations are anatomical landmarks that can guide the safe placement of catheters or needles during medical procedures. Although ultrasound is advantageous in navigating anatomical landmarks in emergency scenarios due to its portability and safety, to our knowledge no existing algorithm can autonomously extract vessel bifurcations using ultrasound images. This is primarily due to the limited availability of ground truth data, in particular, data from live subjects, needed for training and validating reliable models. Researchers often resort to using data from anatomical phantoms or simulations. We introduce BIFURC, Bifurcation Identification for Ultrasound-driven Robot Cannulation, a novel algorithm that identifies vessel bifurcations and provides optimal needle insertion sites for an autonomous robotic cannulation system. BIFURC integrates expert knowledge with deep learning techniques to efficiently detect vessel bifurcations within the femoral region and can be trained on a limited amount of in-vivo data. We evaluated our algorithm using a medical phantom as well as real-world experiments involving live pigs. In all cases, BIFURC consistently identified bifurcation points and needle insertion locations in alignment with those identified by expert clinicians.
Enhanced Uncertainty Estimation in Ultrasound Image Segmentation with MSU-Net
Jul 31, 2024



Abstract:Efficient intravascular access in trauma and critical care significantly impacts patient outcomes. However, the availability of skilled medical personnel in austere environments is often limited. Autonomous robotic ultrasound systems can aid in needle insertion for medication delivery and support non-experts in such tasks. Despite advances in autonomous needle insertion, inaccuracies in vessel segmentation predictions pose risks. Understanding the uncertainty of predictive models in ultrasound imaging is crucial for assessing their reliability. We introduce MSU-Net, a novel multistage approach for training an ensemble of U-Nets to yield accurate ultrasound image segmentation maps. We demonstrate substantial improvements, 18.1% over a single Monte Carlo U-Net, enhancing uncertainty evaluations, model transparency, and trustworthiness. By highlighting areas of model certainty, MSU-Net can guide safe needle insertions, empowering non-experts to accomplish such tasks.
Provably Robust Model-Centric Explanations for Critical Decision-Making
Oct 26, 2021



Abstract:We recommend using a model-centric, Boolean Satisfiability (SAT) formalism to obtain useful explanations of trained model behavior, different and complementary to what can be gleaned from LIME and SHAP, popular data-centric explanation tools in Artificial Intelligence (AI). We compare and contrast these methods, and show that data-centric methods may yield brittle explanations of limited practical utility. The model-centric framework, however, can offer actionable insights into risks of using AI models in practice. For critical applications of AI, split-second decision making is best informed by robust explanations that are invariant to properties of data, the capability offered by model-centric frameworks.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge