Caiwen Jiang
End-to-end Triple-domain PET Enhancement: A Hybrid Denoising-and-reconstruction Framework for Reconstructing Standard-dose PET Images from Low-dose PET Sinograms
Dec 04, 2024
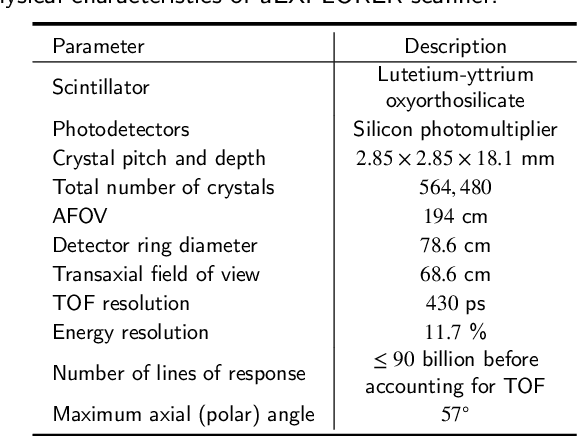

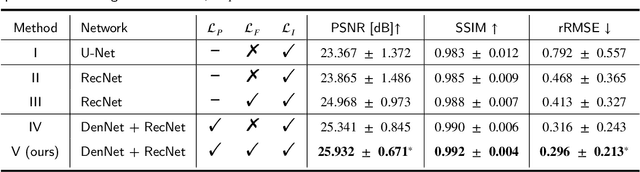
Abstract:As a sensitive functional imaging technique, positron emission tomography (PET) plays a critical role in early disease diagnosis. However, obtaining a high-quality PET image requires injecting a sufficient dose (standard dose) of radionuclides into the body, which inevitably poses radiation hazards to patients. To mitigate radiation hazards, the reconstruction of standard-dose PET (SPET) from low-dose PET (LPET) is desired. According to imaging theory, PET reconstruction process involves multiple domains (e.g., projection domain and image domain), and a significant portion of the difference between SPET and LPET arises from variations in the noise levels introduced during the sampling of raw data as sinograms. In light of these two facts, we propose an end-to-end TriPle-domain LPET EnhancemenT (TriPLET) framework, by leveraging the advantages of a hybrid denoising-and-reconstruction process and a triple-domain representation (i.e., sinograms, frequency spectrum maps, and images) to reconstruct SPET images from LPET sinograms. Specifically, TriPLET consists of three sequentially coupled components including 1) a Transformer-assisted denoising network that denoises the inputted LPET sinograms in the projection domain, 2) a discrete-wavelet-transform-based reconstruction network that further reconstructs SPET from LPET in the wavelet domain, and 3) a pair-based adversarial network that evaluates the reconstructed SPET images in the image domain. Extensive experiments on the real PET dataset demonstrate that our proposed TriPLET can reconstruct SPET images with the highest similarity and signal-to-noise ratio to real data, compared with state-of-the-art methods.
CIResDiff: A Clinically-Informed Residual Diffusion Model for Predicting Idiopathic Pulmonary Fibrosis Progression
Aug 05, 2024


Abstract:The progression of Idiopathic Pulmonary Fibrosis (IPF) significantly correlates with higher patient mortality rates. Early detection of IPF progression is critical for initiating timely treatment, which can effectively slow down the advancement of the disease. However, the current clinical criteria define disease progression requiring two CT scans with a one-year interval, presenting a dilemma: a disease progression is identified only after the disease has already progressed. To this end, in this paper, we develop a novel diffusion model to accurately predict the progression of IPF by generating patient's follow-up CT scan from the initial CT scan. Specifically, from the clinical prior knowledge, we tailor improvements to the traditional diffusion model and propose a Clinically-Informed Residual Diffusion model, called CIResDiff. The key innovations of CIResDiff include 1) performing the target region pre-registration to align the lung regions of two CT scans at different time points for reducing the generation difficulty, 2) adopting the residual diffusion instead of traditional diffusion to enable the model focus more on differences (i.e., lesions) between the two CT scans rather than the largely identical anatomical content, and 3) designing the clinically-informed process based on CLIP technology to integrate lung function information which is highly relevant to diagnosis into the reverse process for assisting generation. Extensive experiments on clinical data demonstrate that our approach can outperform state-of-the-art methods and effectively predict the progression of IPF.
A dual-task mutual learning framework for predicting post-thrombectomy cerebral hemorrhage
Aug 01, 2024Abstract:Ischemic stroke is a severe condition caused by the blockage of brain blood vessels, and can lead to the death of brain tissue due to oxygen deprivation. Thrombectomy has become a common treatment choice for ischemic stroke due to its immediate effectiveness. But, it carries the risk of postoperative cerebral hemorrhage. Clinically, multiple CT scans within 0-72 hours post-surgery are used to monitor for hemorrhage. However, this approach exposes radiation dose to patients, and may delay the detection of cerebral hemorrhage. To address this dilemma, we propose a novel prediction framework for measuring postoperative cerebral hemorrhage using only the patient's initial CT scan. Specifically, we introduce a dual-task mutual learning framework to takes the initial CT scan as input and simultaneously estimates both the follow-up CT scan and prognostic label to predict the occurrence of postoperative cerebral hemorrhage. Our proposed framework incorporates two attention mechanisms, i.e., self-attention and interactive attention. Specifically, the self-attention mechanism allows the model to focus more on high-density areas in the image, which are critical for diagnosis (i.e., potential hemorrhage areas). The interactive attention mechanism further models the dependencies between the interrelated generation and classification tasks, enabling both tasks to perform better than the case when conducted individually. Validated on clinical data, our method can generate follow-up CT scans better than state-of-the-art methods, and achieves an accuracy of 86.37% in predicting follow-up prognostic labels. Thus, our work thus contributes to the timely screening of post-thrombectomy cerebral hemorrhage, and could significantly reform the clinical process of thrombectomy and other similar operations related to stroke.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge